Highlights
- •Oral, intraperitoneal and intratumoral β-glucans inhibited S-180 tumor growth.
- •β-glucans targeted tumors and interacted with tumor cells.
- •β-glucans promoted CD4+ T cell differentiation and neutrophil infiltration.
Abstract
β-glucans are polysaccharides comprising β-D-glucoses with various bioactivities. Herein, we extracted three β-glucans from Lentinus edodes with different sources and assessed their antitumor activities on a mice model with intragastric, intraperitoneal and intratumoral injection. Three polysaccharides were shown to have the same chemical structure of β-(1,3)-glucan with β-(1,6) branches, and exhibited S-180 tumor-suppressing ability with good safety. It was found that β-glucans up-regulated CD4+ T cell level in lymphoid organs decreased by tumor-burden, indicating promotion of immunomodulation. β-glucans targeted tumors in vivo even after oral or intraperitoneal injection. Furthermore, β-glucans not only targeted to lymphoid organs and increased CD4+ T cells number, but also enhanced CD4+ T cells and neutrophils populations in tumors. It was proposed that β-glucans promoted CD4+ T cell immunomodulation and neutrophils infiltration into tumors, leading to tumor growth inhibition. These findings reveal that β-glucans can be used as an effective agent for cancer immunotherapy.
Graphical abstract

Keywords
β-glucan
Antitumor
CD4+ T cell
Neutrophil
1. Introduction
Cancer is still one of the leading causes of death in the world, and great efforts have been made to cure it. Chemotherapy, a type of cancer treatment that uses one or more anti-cancer drugs to kill tumor cells, has long been entrusted for its high efficiency and systemic function (Chabner & Roberts, 2005). But the side effects of the cytotoxic drugs urge the emergence of new therapeutic strategy. In recent years, immunotherapy has become of great interest to researchers, clinicians and pharmaceutical companies, particularly in its promise to treat various forms of cancer with fewer side effects than existing drugs, in which immune effector cells such as lymphocytes, macrophages, dendritic cells, neutrophils and natural killer (NK) cells work together to defend the body against cancer by targeting abnormal antigens expressed on the surface of tumor cells (Syn, Teng, Mok, & Soo, 2017). Current approaches to cancer immunotherapy such as vaccine administration, adoptive cell therapies, and cytokine administration are aimed at stimulating T cells to recognize cancer antigens and develop effector mechanisms that can destroy cancer cells (Restifo, Dudley, & Rosenberg, 2012). Tumor-infiltrating T cells or T cells genetically engineered with antitumor T cell receptors (TCRs) exhibit antitumor activity, which can be adoptively transferred into patients with cancer, thus arming the patients to fight cancer with their own immune system (Rosenberg & Restifo, 2015). Nowadays, growing numbers of strategies that activate antitumor T cells straightway in vivo are applied, such as the cytotoxic T lymphocyte antigen 4 (CTLA4) inhibitor, which blocks immunoinhibitory signals and enables patients to produce an effective antitumor response, yet been approved by FDA for the adjuvant treatment of stage III melanoma and the treatment of advanced melanoma in recent years (Prieto et al., 2012).
CD4+ T cells, also known as T helper cells (Th cells), play an important role in the immune system, particularly in the adaptive immune system. Additionally, CD4+ T cells alone, have been demonstrated to eliminate tumor cells. Adoptive transfer experiments using primed CD4+ T cells generated by immunization with tumor cells confer protection against a subsequent tumor challenge (Muranski & Restifo, 2009). Thus, CD4+ T cells play an important role in modulating immune responses to pathogens and tumor cells, and are important in orchestrating overall immune responses (Kennedy & Celis, 2008). Therefore, it is of great significance to elevate CD4+ T cells level and produce infiltrating CD4+ T cells in antitumor responses.
β-glucans, mostly from the cell wall of cereals, bacteria and fungi, are polymers of glucoses linked by β-glycosidic bonds, mostly with β-1,3-D-backbone and β-1,6-branched glucose residues, known to possess significant biological and physiological activities and can activate the immune system (Goodridge, Wolf, & Underhill, 2009). Unlike chemotherapy drugs, β-glucans achieve good therapeutic effects with relatively low toxicities and few side effects in various diseases, particularly in cancer (Wasser, 2002; Zhang, Cui, Cheung, & Wang, 2007). The β-glucan isolated from an edible mushroom, Lentinus edodes, was first found to act as an antitumor agent against sarcoma 180 in 1969, and has been applied in clinical anticancer therapy in Japan (Chihara, Maeda, Hamuro, Sasaki, & Fukuoka, 1969; Chihara, Hamuro, Maeda, Arai, & Fukuoka, 1970). However, they have not been permitted for use in clinics worldwide due to the undefined pharmacological mechanism. To our best knowledges, intraperitoneal injection of polysaccharides is the usually used drug administration in most reported studies. In our previous work, we have demonstrated that the highly purified β-glucan from Lentinus edodes inhibits S-180 tumor growth by intraperitoneally administration at a low dose of 1 mg/kg (Xu, Zou, Xu, & Zhang, 2016; Zheng, Lu, Xu, & Zhang, 2017).
In this study, we further expanded the antitumor bioactivity, with the BALB/c S-180 tumor model, which is long-established due to its highly aggressive in all strains of laboratory mice and rats (Cui et al., 2003). We investigated the tumor-regressing ability of β-glucan by different modes of administration, and established the connection between β-glucan and CD4+ T cells in tumor immune responses.
2. Materials and methods
2.1. Preparation of β-glucans
Three kinds of dried mushrooms named as Lentinus edodes, planted in autumn, in spring, and cultivated in greenhouse, were provided by Yandi Agricultural Science and Technology Co., Ltd (Hubei, China), and the β-glucans were prepared respectively as previously described (Xu, Chen, Zhang, & Ashida, 2011). Briefly, the fragmentized mushrooms were refluxed in ethyl acetate and acetone for 8 h, and then the residues were dispersed in 0.9% sodium chloride to remove soluble components. The residues were immerged in hot water (121 °C, 0.1 MPa, 30 min) and then in 1.25 M sodium hydroxide/0.05% sodium borohydride (room temperature, 4 h). The supernatant was precipitated with 1 M acetic acid to remove α-glucans, which was then subjected to the 717 type anion exchange resins to remove proteins, followed by treatment with 30% H2O2 to decolorize the solution (Xu et al., 2016). The decolorized solution was dialyzed against ultrapure water with cellulose membranes (Mw cutoff 8000) to remove molecules with relatively low molecular weight. Solutions were then concentrated by rotary evaporation, followed by precipitation into acetone. The precipitates were re-dissolved in pure water, dialysed and lyophilized. Thus, three alkali-extracted β-glucans were yielded and labeled as AG (from autumn-planting mushroom), SG (from spring-planting mushroom) and CG (from cultivation-planting mushroom), respectively.
AG, SG and CG were labeled with fluorescein isothiocyanate (FITC) for tracing (Cao et al., 2016). Briefly, β-glucan (200 mg), FITC (28 mg, Sigma, US), pyridine (80 μL) and dibutyltin dilaurate (16 μL) were first dissolved in dimethyl sulfoxide (DMSO, 20 mL). The reaction mixture was heated in the dark for 4 h at 100 °C and precipitated with 4 volumes of isopropanol by centrifugation (5000 rpm, 20 min, room temperature). The precipitation was repeated four times to remove the unbound FITC. The final samples were obtained after dialysis against ultrapure water and freeze-drying, coded as FITC-AG, FITC-SG and FITC-CG.
Sample AG was also labeled with a Cyanine 5.5 (Cy5.5) dye (Zhang & Kim, 2012). Briefly, 100 mg of AG was dissolved in 100 mL ultrapure water, followed by successively adding 100 mL ethanediamine slowly and 300 mg sodium borohydride, and stirring for 3 days at room temperature. The reaction mixture was precipitated with 300 mL ethanol by centrifugation (5000 rpm, 20 min, room temperature). The precipitates were washed twice with ethanol, dialysed against ultrapure water and freeze-dried. 10 mg lyophilized aminated AG was dissolved in 2 mL phosphate buffer (pH = 8.0), and mixed with 1 mg Cy5.5-NHS ester (Apexbio, US) in 100 μL dimethyl formamide, stirring in the dark overnight at 4 °C. The reaction mixture was precipitated with 4 volumes of isopropanol by centrifugation (5000 rpm, 20 min, room temperature) and washed three times. The final sample was obtained after dialysis against ultrapure water and freeze-drying, coded as Cy5.5-AG.
2.2. Structural characterization of samples
Nuclear magnetic resonance (NMR) Spectroscopy. 13C NMR spectra of AG, SG and CG β-glucans were acquired at 25 °C on a DRX-Bruker spectrometer (Bruker, Germany) operating at a proton frequency of 500 MHz.
Fourier transform infrared spectroscopy (FT-IR). FT-IR spectra of β-glucans were recorded on a Nicolet 170SX FT-IR spectrometer (Spectrum One, PerkinElmer Co., US) in the wavenumber range of 4000-400 cm−1. Samples were dried prior to tableting with KBr powder.
Atom force microscopy (AFM). AFM was performed on a PicoScan 2500 PicoSPM II Controller (Molecular Imaging, US) in a MAC mode with commercial MAC lever TT tips (Molecular Imaging, US), with a spring constant of 1.7 N/m. The sample solution was cast on the freshly cleaved mica and dried overnight before observation. Tapping mode AFM measurements were conducted in air using a nanoscope system operated under ambient conditions with standard silicon tips.
The weight-average molecular weights (Mw) of samples in water were determined using size-exclusion chromatography combined with multi-angle laser light scattering (SEC-MALLS) equipped with a He-Ne laser at λ = 633 nm (DAWN DSP, Wyatt Technology Co., USA) and a differential refractive index detector (DAWN DSP, Wyatt Technology Co., USA) at 25 °C. A shodex-OHpak SB-806 MHQ (8.0 mm × 300 mm) column was used with pure water as the elution solution. The specific refractive index increment (dn/dc) of 0.140 mL/g for polysaccharides in water at 633 nm and 25 °C was adopted (Zhang, Xu, Xu, & Zhang, 2007). The data were collected and analyzed by using the Astra software.
2.3. Mice and cell line
Six-week-old female BALB/c mice were purchased from the Animal Experiment Center of Wuhan University (Wuhan, China), and all animal protocols were approved by the Institutional Animal Care and Use Committee (IACUC) of Wuhan University. Mice were housed under the controlled room temperature (22 ± 2 °C) and humidity (55 ± 5%) on a 12 h light-dark cycle with free access to water and diets.
Mouse sarcoma 180 cell line (S-180) was purchased from China Center for Typical Culture Collection (Wuhan, China) and maintained in RPMI 1640 culture medium (Gibco, US) supplemented with 10% fetal bovine serum (FBS) (Gibco, US) at 37 °C under a humidified atmosphere of 95% air and 5% CO2.
2.4. Tumor models
S-180 cells (3 × 106 cells, 0.2 mL) were intraperitoneally injected into the abdominal cavity of a mouse. After 1 week, the S-180 ascites were drawn and washed with cold phosphate-buffered saline (PBS), then resuspended in saline. Mice were subcutaneously inoculated with ascetic S-180 cells (5 × 106 cells, 0.2 mL) into the right armpit. Daily treatment with intraperitoneal injection of saline (control), Cytoxan (20 mg/kg, Sigma, US) or β-glucans (1 mg/kg) began after tumor cell inoculation for 1 week. Mice were sacrificed after treatment for 2 weeks, and tumors, spleens and thymuses were collected. Spleen and thymus indexes were calculated using the following formula: % = spleen/thymus weight (mg) / body weight (g) × 10.
In some experiments, mice were daily treated with 2 mg/kg AG by intragastric (i.g.) gavage, intraperitoneal (i.p.) or intratumoral (i.t.) injection for 3 weeks. Tumor sizes were measured every two days with a caliper following the day of initial treatment, and the tumor volume was calculated using the following formula: mm3 = (longest diameter × shortest diameter2)/2.
2.5. Lymphocytes preparation
Spleens and thymuses were isolated from healthy or tumor-bearing mice. The tissues were cut into small pieces and grinded gently with a 5-mL syringe plunger on a 200-mesh (75 μm) nylon filtration fabric inside a 6-well plate containing 1 mL cold PBS. The cell suspensions were then passed through the strainer and centrifuged at 2000 rpm for 5 min at 4 °C. The cell pellets were resuspended in 6 mL 40% percoll, and softly added to the top of 3 mL 70% percoll in 15-mL tubes. The binary mixture was centrifuged at 400 g for 20 min at room temperature, and lymphocytes were harvested from the interlayer and washed with cold PBS.
2.6. Flow cytometry
Lymphocytes from spleens and thymuses of healthy or S-180 tumor-bearing mice were harvested and washed with PBS, then stained with rat-anti-mouse FITC-conjugated anti-CD3, APC-conjugated anti-CD4, FITC-conjugated anti-CD8a or corresponding fluorescein-conjugated isotype mAb (Invitrogen, US). After incubation at room temperature for 40 min and washed with PBS three times, cell suspensions in 500 μL buffer were loaded to a BD FACSVerse™ flow cytometer (BD Biosciences, US) and data were acquired by BD FACSuite Software and analyzed by FlowJo.
In some cases, S-180 cells were collected and incubated with FITC-labeled β-glucan in serum-free RPMI 1640 medium at 4 °C or 37 °C for 2 h. After washed with PBS three times and resuspended in 500 μL buffer, fluorescence signal of the cells was measured on a flow cytometer.
2.7. Confocal microscopy
S-180 cells were incubated with FITC-labeled β-glucan in serum-free RPMI 1640 medium at 4 °C or 37 °C for 2 h and stained with Hoechst 33342 dye at room temperature for 10 min. After washed with PBS three times and resuspended in PBS at a density of 5 × 105 /mL, 100 μL of the cell suspension was dropwise added onto the glass bottom of a confocal dish and covered with a coverslip. Images were acquired under an oil immersion objective at 60× magnification on UltraVIEW VoX confocal microscope (Perkin-Elmer, US).
2.8. Immunofluorescence assay of tissues
Tumors and spleens were isolated from tumor-bearing mice treated with oral FITC-AG or Cy5.5-AG. Then cryosections of fresh tissues were stained with 4,6-diamidino-2-phenylindole (DAPI, Invitrogen, US) counterstaining to detect the biodistribution of AG. Fluorescence scanning was manipulated on Pannoramic MIDI (3D HISTECH, Hungary).
In some cases, tumors from AG-treated mice were fixed in 4% paraformaldehyde overnight and embedded in paraffin. Tumor slides were incubated overnight with biotin-conjugated antibodies including anti-F4/80, anti-Ly-6 G, anti-CD4 and anti-CD49b (Invitrogen, US), then streptavidin-FITC or streptavidin-Cy3 (Invitrogen, US), and counterstained with DAPI.
2.9. In vivo living fluorescent imaging
Cy5.5-AG (10 mg/kg) was administrated orally, intraperitoneally or intratumorally into S-180 tumor-bearing mice shaved in the front. Fluorescent images were captured on a living image IVIS® spectrum (Perkin-Elmer, US) at different time points.
2.10. MTT assay
S-180 cells were seeded in 96-well plates at a concentration of 5 × 103 cells/well in a final volume of 200 μL and incubated for 24 h. The cells were then incubated with PBS or β-glucan samples dissolved in the complete cell culture medium at final concentrations of 50, 100 or 200 μg/mL. After a 24-h incubation, 10 μL of 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide (MTT, 5 mg/mL, Sigma, US) solution was added to each well and incubated at 37 °C for 4 h. After centrifugation, the supernatant was removed, and the precipitates were treated with 200 μL of DMSO, followed by gently shaking. The absorbance of the plates was analyzed at a wavelength of 570 nm on a Tecan Spark™ 10 M microplate reader (Tecan, Switzerland). The data was presented as cell viability (%) = β-glucan-treated group / PBS control group × 100%.
2.11. Statistics
All data are presented as the mean ± standard error (S.E.) of at least three independent experiments unless specified otherwise. Student’s t-test was performed and the differences were considered statistically significant at p < 0.05.
3. Results and discussion
3.1. Chemical structures and molecular weights of β-glucans
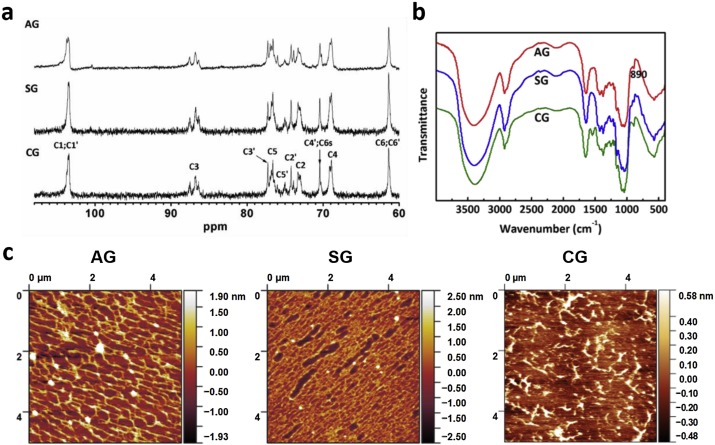
Our previous study has shown that the polysaccharide from the mushroom of Lentinus edodes through NaOH extraction is a β-(1,3)-glucan with β-(1,6)-glucose residues as branches (Li, Chen, Xu, & Xu, 2017). To verify the chemical structure, three polysaccharides of AG, SG and CG were characterized by NMR and FT-IR. Fig. 1a shows the 13C NMR of three samples dissolved in DMSO. Three samples displayed the similar spectra with the anomeric carbon (C1) of 103.6 ppm and C3 of 86.8 ppm, indicating the β-(1,3)-linkage (Wang & Zhang, 2009). Chemical shifts of other carbons were also assigned and summarized in Table S1, consistent with the data of our previously reported lentinan sample (Li et al., 2017), that is, the three polysaccharides were β-1,3-glucans with side branches of (1,6) glucosyl units, almost independent of planting seasons and the cultivation modes. FT-IR spectra confirmed the β-configuration of the samples with the characteristic absorption at 890 cm−1 (Fig. 1b).

The weight-average molecular weights (Mw) of AG, SG and CG in water were estimated to be 9.5 × 105, 1.7 × 106 and 7.9 × 105 with SEC-MALLS. Clearly, the molecular weight was dependent on the planting season and cultivation mode; of these, SG from the spring-planting mushrooms possessed the highest molecular weight, and the sample CG from the cultivated mushrooms had the lowest molecular weight possibly due to the shortest growth cycle of cultivation.
Fig. 1c shows the AFM images of AG, SG and CG samples which were first dissolved in water with the same concentration of 5 μg/mL followed by drying at room temperature. AG and SG existed as network-like shape, and SG adopted a denser network than AG. By contrast, CG did not form network, but mainly existed as random aggregates. In our previous work, it has been demonstrated that the triple helical polysaccharide with relatively low molecular weight forms “faggot-like” aggregates due to parallel accumulation of short polysaccharide chains (Zhang, Li, & Zhang, 2010). By contrast, the long chains of polysaccharides with high molecular weights are easily entangled to form network-like structure at relatively high concentrations. For example, lentinan with Mw of 1.6 × 106 in water formed gel with a dense network structure observed by AFM image (Zhang, Xu, & Zhang, 2008). In combination, these different morphologies in the three samples were mainly ascribed to the different molecular weights as they share almost the same chemical structure according to the 13C NMR and FT-IR spectra.
3.2. Inhibition of S-180 tumor growth by β-glucans
According to our previous study, β-1,3-glucan from Lentinus edodes can inhibit the growth of mice S-180 tumors when intraperitoneally administrated (Xu et al., 2016). Herein, we used three β-glucans with different molecular weights from different mushrooms to further confirm the antitumor activity on mice S-180 tumor model by intraperitoneal administration. Fig. 2a shows that all β-glucans decreased the S-180 tumor weights to a noteworthy level with a dosage of 1 mg⋅ kg−1⋅day−1, especially AG with a molecular weight of 9.5 × 105 from the autumn-planting mushrooms achieved the level of positive drug Cytoxan (20 mg⋅ kg−1⋅day−1), with an inhibition ratio of 58% (Fig. 2b). It is noteworthy that the dosage of positive drug of Cytoxan was 20 times as much as those of β-glucans, indicative of high efficacy of β-glucans. Besides, all β-glucan-treated mice showed retained spleen and thymus indexes akin to the control group (Fig. 2c and d), while Cytoxan strongly decreased the spleen and thymus indexes, showing good safety of β-glucan and strong toxicity of Cytoxan. These results are consistent with our previously reported data (Xu et al., 2016), further demonstrating the feasibility of β-glucans as antitumor drugs. It is noted that sample AG showed the higher inhibition ratio of tumor growth than SG and CG. As reported, immunomodulation plays an important role in the antitumor of polysaccharides (Wasser, 2002). Osorio et al have demonstrated that the recognition of β-glucans by the receptor on the surface of immune cells triggers the downstream signal responses, leading to the immunomodulation (Osorio & Reis e Sousa, 2011). Furthermore, polysaccharides with low molecular weights or small size have less chance to interact with the receptor on the cell surface and to trigger the downstream signal responses than those with high molecular weight or big size (Adams et al., 2008; Goodridge et al., 2011). As discussed in the first part, the polysaccharides with longer chains were easily entangled to form a denser network by themselves, decreasing the chance to interact with the receptor. In combination with these reported results and our data, it can be proposed that the polysaccharide of AG with the medium molecular weight would have the stronger interaction with the receptor on the cell surface, leading to its higher tumor inhibition than SG with the longer chain and CG with the shorter chain. In the following, sample AG was focused on for further study.

Since oral medication is a more convenient and low-cost way in treatment of disease, which is seldom reported for polysaccharides in tumor treatment, we adopted an additional mode of administration by i.g. gavage in this study. Fig. 3 shows that AG administrated by i.p. or by i.g. both restrained the growth of S-180 tumors. It is worth noting that the i.g. gavage of AG led to the highest inhibition of tumor growth (Fig. 3e). There are reports that show the rapid and efficient intestinal absorption of β-glucan (Rice et al., 2005), followed by binding to and internalization into gut-associated lymphoid tissue cells, thereby translocating itself into the systemic circulation to produce significant biological effects. Therefore, i.g. gavage of polysaccharides is really a feasible administration mode, and the antitumor mechanism is deserved for further investigation.

3.3. β-Glucan treatment reverses the lymphocytes change caused by tumor burden
Spleen and thymus are peripheral and primary immune organs that participate in numerous immune modulation responses like anti-tumor and anti-inflammation. Spleen and thymus indexes, which depend on the lymphopoiesis of the organs, are rough and general indicators of the immunity. β-glucan is generally considered as an immune modulator rather than a direct killer of tumors like synthetic anticancer drugs (Chan, Chan, & Sze, 2009). Herein, we thus focused on the immunomodulation of β-glucans from Lentinus edodes in mice bearing tumors, and examined the lymphocytes in spleen and thymus for better understanding the effect of β-glucan on immunity in vivo. As a result, compared with the normal group, S-180 tumor burden (the control group) decreased the proportion of T cells, and i.p. or i.g. administration of 2 mg/kg AG daily hardly changed the T cell level distinctly (data not shown). However, further analysis on CD3+CD4+ cells revealed that i.p. or i.g administration of AG could restore the decrease of CD4+ proportion in CD3+ T cells caused by tumor burdens (Fig. 4a). As well known, CD4+ T cells play an important role in modulating immune responses to pathogens and tumor cells, and are important in orchestrating overall immune responses (Kennedy & Celis, 2008). In our findings, the decrease of CD4+ proportion in T cells from tumor-bearing mice was observed, and AG treatment restored the decrease of CD4+ proportion in CD3+ T cells, which may contribute to the antitumor effect.

It is popularly known that T lymphocytes originate from bone marrow lymphoid stem cells that differentiate and mature into T lymphocytes in thymus, followed by migrating into immune organs and tissues through lymph and blood circulation for immunomodulation. We thus check the effect of β-glucans on the T lymphocytes in the thymus. As a result, tumor burden hardly altered the lymphocyte quantity or T cell component (data not shown), but sharply decreased the single positive CD4+ T cells number (Fig. 4b, control). Notably, β-glucans significantly increased CD4+CD3– cells number in the thymus (Fig. 4b). There are studies showing that CD4+CD3– cells have two functions, a well-established role in organizing lymphoid tissue during development, and a later discovered role in supporting T-cell help for B cells both during affinity maturation in germinal centres and for memory antibody responses (Kim et al., 2003; Mebius, 2003). Therefore, β-glucans may help maintaining the lymphoid organs as well as supporting adaptive memory antibody responses which is disturbed by tumors through increasing CD4+CD3– cells number. In a word, S-180 tumor burden reduced immune-associated T helper cells in spleen and thymus, and β-glucan restored the T cell subset imbalance somehow.
3.4. Orally administered β-glucan promotes T helper cell differentiation in healthy mice
As a bioactive macromolecule, β-glucan is considered to play an immune-modulating role for fighting against cancers or other diseases. Hence to evaluate the influence of β-glucan on immune cells, we treated healthy mice with AG through oral administration and extracted spleen and thymus lymphocytes. Flow cytometric analysis shows that AG did up-regulate the proportion of CD4+ Th cells in spleen (Fig. 5a, b). This may help maintaining the homeostasis of T cells and preventing tumorigenesis (Ma et al., 2016). No significant changes occurred in thymus lymphocytes (Fig. 5c, d). CD8+ T cells are another group of T cells, which are traditionally thought to be the major mediators of effective antitumor T cell responses and can directly kill the cancerous cells which are specific antigen-reactive (Cui & Kaech, 2010). Spleen and thymus T cells were distinguished by CD4- or CD8-antibodies. Flow cytometric data show no clear difference between spleen T cell subsets of untreated and AG-treated mice (Fig. 5e). However, thymus lymphocytes data reveal that AG treatment increased the proportion of CD4+ T cells, and decreased the amount of CD4+CD8+ double-positive cells, while caused no change in CD8+ T cells (Fig. 5f). T lymphocytes undergo two steps during their development and maturation in thymus. They first become CD4+CD8+ double-positive thymocytes, and finally mature to CD4+CD8− or CD4−CD8+ single-positive CD3+ T cells that are then released from the thymus to peripheral tissues (Schwarz & Bhandoola, 2006). In this study, CD8+CD4– T cells were barely seen in thymus lymphocytes, while CD4+CD8– T cells increased distinctly, suggesting that β-glucan can mature the primary T cells in the thymus and polarize them to T helper cells. In combination of these data, it can be supposed that CD4+ T cells generated in the thymus were transported to peripheral sites including spleen with other subsets in a relatively constant ratio, leading to the enhancement of CD4+ proportion in spleen lymphocytes for enhancing immunomodulation.

3.5. Biodistribution of orally administered β-glucans in tumor and spleen
To clarify the biodistribution of β-glucans in tissues and if they interact with the organs, S-180 tumor-bearing mice were orally treated with FITC-AG. Tumors and spleens were extracted for fluorescence scanning. Fluorescence images marked the traces of AG in tumors or spleens at 2 h post-gavage (Fig. 6a). FITC-AG (green color) distributed itself uniformly all over the tissue in the spleen, overlapping with the nucleus-indicative blue fluorescence. It is surprising that the green fluorescence in a reticulate vein shape in the tumor was observed, which means that β-glucans can reach the tumor site. To our best knowledge, this is almost the first time that the orally administered β-glucans were seen in the tumor site. Studies have demonstrated that oral β-glucans can be taken up by macrophages, transported to spleen (Li et al., 2010) and induces systemic immune responses (Masuda et al., 2013). Besides, β-glucan recognition by dectin-1 will activate dendritic cells and lead to CD4+ T cells maturation and differentiation (LeibundGut-Landmann et al., 2007). In our findings, the β-glucan AG could bind to peritoneal macrophages of mice (Fig. S1), probably through dectin-1 receptor because dectin-1 is demonstrated to express on the surface of macrophages (Taylor et al., 2002). We assumed that the gastrointestinal macrophages took up oral β-glucans and migrated to the spleen (Li et al., 2006), leading to distribution of AG in this tissue. Some researches show that neutrophils take up macrophage-degraded β-glucans (Zheng, Zhou, Xu, & Zhang, 2017) and deliver the β-glucan fragments to tumor cells via the blood (Hong et al., 2004). From the partial enlarged image (Fig. S2), the tumor site showed bright but shattered green fluorescence in vessel-like shapes, meanwhile the nucleus-indicating DAPI presented loose and fractured blue fluorescence, strongly contrast to the fluorescence spreading evenly in the intact spleen parenchyma. It is possible that β-glucans were taken up in macrophages and neutrophils, and were delivered to the tumor site via blood circulation, leading to the destruction of tumor tissues and probably block of the tumor microvessels by AG. As shown in Fig. 6b, FITC-AG was still detectable after 72 h in the intact spleen, demonstrating its durable immunomodulating activity.

3.6. β-Glucan interacts with S-180 cells in vitro
The results above show that AG not only functioned on spleen and thymus and influenced the immune cells, but also targeted S-180 tumor cells. So, we examined the interaction between AG β-glucan and S-180 cells in vitro. Flow cytometry analysis illustrates the binding of FITC-AG to the S-180 cells (Fig. 7a, b). It is widely believed that cells cannot conduct the ATP-dominated phagocytosis at 4 °C, so the FITC-positive signal in the cell group was due to the interaction between AG and S-180 cells, maybe some β-glucan-related receptors on the cell surface. Furthermore, the confocal microscopy of AG-treated S-180 cells confirmed β-glucan binding to the cell surface characterized by the fluorescent circle at 4 °C. Brighter green fluorescence at 37 °C was observed due to the cell phagocytosis of FITC-AG (Fig. 7c). Interestingly, the β-glucans CG and SG with less antitumor effect showed less positive staining to S-180 cells (Fig. 7d–f). We thus speculate that the tumor-suppressing activity may be partially attributed to the interaction between β-glucan and tumor cells in tumor tissues. MTT assay proved a dose-dependent inhibition of AG towards S-180 cells growth (Fig. 7g).

In vitro flow cytometry and cell viability assay showed similar outcome as the in vivo antitumor experiment; that is, AG bound to S-180 cells, and exhibited strongest inhibition against S-180 cells proliferation. It should be noted that, the inhibition of β-glucan against S-180 cells in vitro is not as efficient as that in vivo, indicating that the inhibition of tumor growth in vivo is partially rather than entirely depending on the interaction between β-glucan and tumor cells. The nonnegligible fluorescence at 4 °C in confocal microscopy of AG-treated S-180 cells brought us the possibility of one or some receptors on the S-180 cells that would bind to the β-glucan. Thus, we isolated the membrane proteins binding to AG and analyzed them using proteomic technology (Fig. S3). Mass spectrum identification and comparison in database confirmed that would most likely be Elongation factor 1-alpha 1 (eEF1a1), a protein that connected with cell growth, proliferation and tumorigenesis (Thornton, Anand, Purcell, & Lee, 2003). This protein also act as a membrane receptor for the cryptic antiadhesive site of fibronectin, thus inhibiting cell anchorage and promoting apoptosis, or anoikis (Itagaki et al., 2012). Further study will be made on the relation between β-glucan and eEF1a1 in our future work.
3.7. AG β-glucan administrated by different modes all suppresses S-180 tumor growth and targets tumors
The direct effect of AG β-glucan on S-180 cells brings about the possibility of antitumor function point-to-point. Therefore, we repeated in vivo assay with an extra group of β-glucan administration by i.t. injection. Fig. 8 shows different modes of AG administration all led to S-180 tumor regression, especially the i.t. injection achieved a higher inhibition ratio than other two groups. Tumor-bearing mice were treated with Cy5.5-AG and monitored by IVIS. In vivo fluorescence imaging illustrates that AG-treated mice all showed fluorescence at the tumor site, demonstrating the accumulation of Cy5.5-AG to the tumor region (Fig. 9a–c). The intratumorally AG-treated mice showed the strongest fluorescence, as the β-glucans were compulsively settled to the tumors. And slightly lower fluorescence could be observed in the intraperitoneally or orally treated mice, suggesting certain targetability of β-glucans to the tumor. Besides, there was Cy5.5 fluorescence in the abdominal cavity, mostly in the liver and intestine sites. Fluorescence scanning images of tumors ex vivo also displayed Cy5.5 fluorescence in the tumor tissue (Fig. 9d). The continuous fluorescence of the i.t. injected AG-treated mice suggests the stability and sustainability of AG at the tumor site. This may explain the strongest effect of intratumoral AG inhibition on tumor growth, including the efficiently drug-targeting delivery (Wan et al., 2016).


3.8. AG treatment alters the tumor microenvironment
We have already confirmed that β-glucan inhibits tumor growth partially depending on strengthening the immune system. Thus, some immune cells were inspected at the tumor site and the immunofluorescence images are shown in Fig. 10. It can be seen that CD4+ T cells were present in tumors from AG-treated mice, especially in the i.t. group. As shown above, we have confirmed that β-glucan treatment can up-regulate CD4+ T cells in lymphoid organs. These data suggest that AG could recruit CD4+ T cells to tumor tissues, thus killed the tumor cells (Kawashima et al., 2012). While CD49b+ NK cells couldn’t be found in tumors. F4/80-labeled macrophages could be detected in all tumors, like the distribution of Cy5.5-AG in tumors (Fig. 9d). And no significant difference was observed between groups. Interestingly, Ly-6G-marked neutrophils, which rarely existed in the control group, largely emerged in AG-treated groups. It’s worth noting that inside the tumors of AG intratumorally-injected mice there were a large number of neutrophils almost filling up the tissue. There are also studies showing that neutrophils can kill tumor cells when they bind to β-1,3-glucans (Xia et al., 1999). Intravenously injected β-1,3-glucans could directly bind to complement receptor 3 on neutrophils from peripheral blood and gives them the ability to kill tumor cells (Hong et al., 2004). In our case, the β-glucan AG sustaining at tumor site made the neutrophils infiltrating continuously and brought enormous injury to S-180 tumors. In vivo images of orally or intraperitoneally treated mice showed that the fluorescence concentrated in abdominal cavity as well as the tumor sites. So orally and intraperitoneally administered AG, which were processed by gastrointestinal and peritoneal macrophages, drove neutrophils to the tumor sites and killed tumor cells, despite weaker than intratumorally. Thus, it can be conjectured that β-glucans could recruit neutrophils to the tumor site for killing tumor cells.

4. Conclusion
In summary, three β-glucans (AG, SG, and CG) from Lentinus edodes with different sources were isolated with the same chemical structure and different molecular weights. They all exhibited strong antitumor activity and the AG β-glucan with the highest antitumor bioactivity was chosen to investigate the mechanism of tumor-suppression in vivo. AG administrated orally, intraperitoneally or intratumorally all inhibited tumor-growth and targeted the tumor sites. AG up-regulated the CD4+ T cell level in lymphoid organs to reverse the impact of tumor burden, as well as in healthy mice. AG treatment altered the tumor microenvironment, promoted CD4+ T cell and neutrophil infiltration into tumor sites, drove neutrophils to the tumor site or directly recruits neutrophils from peripheral blood to kill tumors. This study thus offers a better understanding for treating cancer by oral β-glucans or a more effective way by intratumoral administration.
Acknowledgement
We gratefully acknowledge the financial supports from the National Natural Science Foundation of China (21574102, 21875167, and 21274114).
Appendix A. Supplementary data
The following is Supplementary data to this article:Download: Download Word document (1MB)
References
- Adams et al., 2008E.L. Adams, P.J. Rice, B. Graves, H.E. Ensley, H. Yu, G.D. Brown, …, D.L. WilliamsDifferential high-affinity interaction of Dectin-1 with natural or synthetic glucans is dependent upon primary structure and is influenced by polymer chain length and side-chain branchingThe Journal of Pharmacology and Experimental Therapeutics, 325 (1) (2008), pp. 115-123View PDFView articleCrossrefView in ScopusGoogle Scholar
- Cao et al., 2016Y. Cao, S. Zou, H. Xu, M. Li, Z. Tong, M. Xu, …, X. XuHypoglycemic activity of the Baker’s yeast β-glucan in obese/type 2 diabetic mice and the underlying mechanismMolecular Nutrition & Food Research, 60 (12) (2016), pp. 2678-2690CrossrefView in ScopusGoogle Scholar
- Chabner and Roberts, 2005B.A. Chabner, T.G. Roberts Jr.Chemotherapy and the war on cancerNature Reviews Cancer, 5 (1) (2005), pp. 65-72CrossrefView in ScopusGoogle Scholar
- Chan et al., 2009G.C. Chan, W.K. Chan, D.M. SzeThe effects of β-glucan on human immune and cancer cellsJournal of Hematology & Oncology, 2 (1) (2009), pp. 25-35View in ScopusGoogle Scholar
- Chihara et al., 1970G. Chihara, J. Hamuro, Y.Y. Maeda, Y. Arai, F. FukuokaFractionation and purification of the polysaccharides with marked antitumor activity, especially lentinan, from Lentinus edodes (Berk.) Sing. (an Edible Mushroom)Cancer Research, 30 (11) (1970), pp. 2776-2781View in ScopusGoogle Scholar
- Chihara et al., 1969G. Chihara, Y. Maeda, J. Hamuro, T. Sasaki, F. FukuokaInhibition of mouse sarcoma 180 by polysaccharides from Lentinus edodes (Berk.) SingNature, 222 (5194) (1969), pp. 687-688CrossrefView in ScopusGoogle Scholar
- Cui and Kaech, 2010W. Cui, S.M. KaechGeneration of effector CD8+ T cells and their conversion to memory T cellsImmunological Reviews, 236 (1) (2010), pp. 151-166View in ScopusGoogle Scholar
- Cui et al., 2003Z. Cui, M.C. Willingham, A.M. Hicks, M.A. Alexander-Miller, T.D. Howard, G.A. Hawkins, …, C.J. DeLongSpontaneous regression of advanced cancer: Identification of a unique genetically determined, age-dependent trait in miceProceedings of the National Academy of Sciences, 100 (11) (2003), pp. 6682-6687View in ScopusGoogle Scholar
- Goodridge et al., 2011H.S. Goodridge, C.N. Reyes, C.A. Becker, T.R. Katsumoto, J. Ma, A.J. Wolf, …, D.M. UnderhillActivation of the innate immune receptor Dectin-1 upon formation of a ‘phagocytic synapse’Nature, 472 (7344) (2011), pp. 471-476CrossrefView in ScopusGoogle Scholar
- Goodridge et al., 2009H.S. Goodridge, A.J. Wolf, D.M. Underhillβ-glucan recognition by the innate immune systemImmunological Reviews, 230 (1) (2009), pp. 38-50View in ScopusGoogle Scholar
- Hong et al., 2004F. Hong, J. Yan, J.T. Baran, D.J. Allendorf, R.D. Hansen, G.R. Ostroff, …, G.D. RossMechanism by which orally administered β-1,3-glucans enhance the tumoricidal activity of antitumor monoclonal antibodies in murine tumor modelsThe Journal of Immunology, 173 (2) (2004), pp. 797-806CrossrefView in ScopusGoogle Scholar
- Itagaki et al., 2012K. Itagaki, T. Naito, R. Iwakiri, M. Haga, S. Miura, Y. Saito, …, F. FukaiEukaryotic translation elongation factor 1A induces Anoikis by triggering cell detachmentThe Journal of Biological Chemistry, 287 (19) (2012), pp. 16037-16046View PDFView articleCrossrefView in ScopusGoogle Scholar
- Kawashima et al., 2012S. Kawashima, K. Hirose, A. Iwata, K. Takahashi, A. Ohkubo, T. Tamachi, …, H. Nakajimaβ-Glucan Curdlan induces IL-10–producing CD4+ T cells and inhibits allergic airway inflammationThe Journal of Immunology, 189 (12) (2012), pp. 5713-5721CrossrefView in ScopusGoogle Scholar
- Kennedy and Celis, 2008R. Kennedy, E. CelisMultiple roles for CD4+ T cells in anti-tumor immune responsesImmunological Reviews, 222 (1) (2008), pp. 129-144View in ScopusGoogle Scholar
- Kim et al., 2003M. Kim, F.M.C. Gaspal, H.E. Wiggett, F.M. McConnell, A. Gulbranson-Judge, C. Raykundalia, …, P.J.L. LaneCD4+CD3− accessory cells costimulate primed CD4 T cells through OX40 and CD30 at sites where T cells collaborate with B cellsImmunity, 18 (5) (2003), pp. 643-654View PDFView articleView in ScopusGoogle Scholar
- LeibundGut-Landmann et al., 2007S. LeibundGut-Landmann, O. Groß, M.J. Robinson, F. Osorio, E.C. Slack, S.V. Tsoni, …, C. Reis e SousaSyk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17Nature Immunology, 8 (6) (2007), pp. 630-638CrossrefView in ScopusGoogle Scholar
- Li et al., 2006B. Li, D.J. Allendorf, R. Hansen, J. Marroquin, C. Ding, D.E. Cramer, …, J. YanYeast β-glucan amplifies phagocyte killing of iC3b-opsonized tumor cells via complement receptor 3-Syk-phosphatidylinositol 3-kinase pathwayThe Journal of Immunology, 177 (3) (2006), pp. 1661-1669CrossrefView in ScopusGoogle Scholar
- Li et al., 2010B. Li, Y. Cai, C. Qi, R. Hansen, C. Ding, T. Mitchell, …, J. YanOrally administered particular β-Glucan modulates tumor-capturing dendritic cells and improves anti-tumor T cell responses in cancerClinical Cancer Research, 16 (21) (2010), pp. 5153-5164View in ScopusGoogle Scholar
- Li et al., 2017M. Li, P. Chen, M. Xu, X. XuA novel self-assembly Lentinan-tetraphenylethylene composite with strong blue fluorescence in water and its propertiesCarbohydrate Polymers, 174 (2017), pp. 13-24View PDFView articleView in ScopusGoogle Scholar
- Ma et al., 2016C. Ma, A.H. Kesarwala, T. Eggert, J. Medina-Echeverz, D.E. Kleiner, P. Jin, …, T.F. GretenNAFLD causes selective CD4+ T lymphocyte loss and promotes hepatocarcinogenesisNature, 531 (7593) (2016), pp. 253-257CrossrefView in ScopusGoogle Scholar
- Masuda et al., 2013Y. Masuda, H. Inoue, H. Ohta, A. Miyake, M. Konishi, H. NanbaOral administration of soluble β-glucans extracted from Grifola frondosa induces systemic antitumor immune response and decreases immunosuppression in tumor-bearing miceInternational Journal of Cancer, 133 (1) (2013), pp. 108-119CrossrefView in ScopusGoogle Scholar
- Mebius, 2003R.E. MebiusOrganogenesis of lymphoid tissuesNature Reviews Immunology, 3 (4) (2003), pp. 292-303CrossrefView in ScopusGoogle Scholar
- Muranski and Restifo, 2009P. Muranski, N.P. RestifoAdoptive immunotherapy of cancer using CD4+ T cellsCurrent Opinion in Immunology, 21 (2) (2009), pp. 200-208View PDFView articleView in ScopusGoogle Scholar
- Osorio and Reis e Sousa, 2011F. Osorio, C. Reis e SousaMyeloid C-type lectin receptors in pathogen recognition and host defenseImmunity, 34 (5) (2011), pp. 651-664View PDFView articleView in ScopusGoogle Scholar
- Prieto et al., 2012P.A. Prieto, J.C. Yang, R.M. Sherry, M.S. Hughes, U.S. Kammula, D.E. White, …, G.Q. PhanCTLA-4 blockade with ipilimumab: Long-term follow-up of 177 patients with metastatic melanomaClinical Cancer Research, 18 (7) (2012), pp. 2039-2047View in ScopusGoogle Scholar
- Restifo et al., 2012N.P. Restifo, M.E. Dudley, S.A. RosenbergAdoptive immunotherapy for cancer: Harnessing the T cell responseNature Reviews Immunology, 12 (4) (2012), pp. 269-281CrossrefView in ScopusGoogle Scholar
- Rice et al., 2005P.J. Rice, E.L. Adams, T. Ozment-Skelton, A.J. Gonzalez, M.P. Goldman, B.E. Lockhart, …, D.L. WilliamsOral delivery and gastrointestinal absorption of soluble glucans stimulate increased resistance to infectious challengeThe Journal of Pharmacology and Experimental Therapeutics, 314 (3) (2005), pp. 1079-1086View PDFView articleCrossrefView in ScopusGoogle Scholar
- Rosenberg and Restifo, 2015S.A. Rosenberg, N.P. RestifoAdoptive cell transfer as personalized immunotherapy for human cancerScience, 348 (6230) (2015), pp. 62-68CrossrefView in ScopusGoogle Scholar
- Schwarz and Bhandoola, 2006B.A. Schwarz, A. BhandoolaTrafficking from the bone marrow to the thymus: A prerequisite for thymopoiesisImmunological Reviews, 209 (1) (2006), pp. 47-57CrossrefView in ScopusGoogle Scholar
- Syn et al., 2017N.L. Syn, M.W.L. Teng, T.S.K. Mok, R.A. SooDe-novo and acquired resistance to immune checkpoint targetingThe Lancet Oncology, 18 (12) (2017), pp. e731-e741View PDFView articleView in ScopusGoogle Scholar
- Taylor et al., 2002P.R. Taylor, G.D. Brown, D.M. Reid, J.A. Willment, L. Martinez-Pomares, S. Gordon, …, S.Y.C. WongThe β-glucan receptor, Dectin-1, is predominantly expressed on the surface of cells of the monocyte/macrophage and neutrophil lineagesThe Journal of Immunology, 169 (7) (2002), pp. 3876-3882CrossrefView in ScopusGoogle Scholar
- Thornton et al., 2003S. Thornton, N. Anand, D. Purcell, J. LeeNot just for housekeeping: Protein initiation and elongation factors in cell growth and tumorigenesisJournal of Molecular Medicine, 81 (9) (2003), pp. 536-548View in ScopusGoogle Scholar
- Wan et al., 2016J. Wan, S. Geng, H. Zhao, X. Peng, Q. Zhou, H. Li, …, H. XuDoxorubicin-induced co-assembling nanomedicines with temperature-sensitive acidic polymer and their in-situ-forming hydrogels for intratumoral administrationJournal of Controlled Release, 235 (2016), pp. 328-336View PDFView articleView in ScopusGoogle Scholar
- Wang and Zhang, 2009X. Wang, L. ZhangPhysicochemical properties and antitumor activities for sulfated derivatives of lentinanCarbohydrate Research, 344 (16) (2009), pp. 2209-2216View PDFView articleView in ScopusGoogle Scholar
- Wasser, 2002S. WasserMedicinal mushrooms as a source of antitumor and immunomodulating polysaccharidesApplied Microbiology and Biotechnology, 60 (3) (2002), pp. 258-274View in ScopusGoogle Scholar
- Xia et al., 1999Y. Xia, V. Větvička, J. Yan, M. Hanikýřová, T. Mayadas, G.D. RossThe β-glucan-binding lectin site of mouse CR3 (CD11b/CD18) and its function in generating a primed state of the receptor that mediates cytotoxic activation in response to iC3b-opsonized target cellsThe Journal of Immunology, 162 (4) (1999), pp. 2281-2290CrossrefGoogle Scholar
- Xu et al., 2016H. Xu, S. Zou, X. Xu, L. ZhangAnti-tumor effect of β-glucan from Lentinus edodes and the underlying mechanismScientific Reports, 6 (2016), p. 28802View in ScopusGoogle Scholar
- Xu et al., 2011X. Xu, P. Chen, L. Zhang, H. AshidaImmunomodulatory β-glucan from Lentinus edodes activates mitogen-activated protein kinases and nuclear factor-κB in Murine RAW 264.7 macrophagesThe Journal of Biological Chemistry, 286 (36) (2011), pp. 31194-31198View PDFView articleView in ScopusGoogle Scholar
- Zhang, Cui et al., 2007M. Zhang, S.W. Cui, P.C.K. Cheung, Q. WangAntitumor polysaccharides from mushrooms: A review on their isolation process, structural characteristics and antitumor activityTrends in Food Science & Technology, 18 (1) (2007), pp. 4-19View PDFView articleView in ScopusGoogle Scholar
- Zhang and Kim, 2012M. Zhang, J.A. KimEffect of molecular size and modification pattern on the internalization of water soluble β-(1→3)-(1→4)-glucan by primary murine macrophagesThe International Journal of Biochemistry & Cell Biology, 44 (6) (2012), pp. 914-927View PDFView articleView in ScopusGoogle Scholar
- Zhang et al., 2010Y. Zhang, S. Li, L. ZhangAggregation behavior of triple helical polysaccharide with low molecular weight in diluted aqueous solutionThe Journal of Physical Chemistry B, 114 (15) (2010), pp. 4945-4954CrossrefView in ScopusGoogle Scholar
- Zhang, Xu et al., 2007Y. Zhang, X. Xu, J. Xu, L. ZhangDynamic viscoelastic behavior of triple helical Lentinan in water: Effects of concentration and molecular weightPolymer, 48 (22) (2007), pp. 6681-6690View PDFView articleView in ScopusGoogle Scholar
- Zhang et al., 2008Y. Zhang, X. Xu, L. ZhangGel formation and low-temperature intramolecular conformation transition of a triple-helical polysaccharide lentinan in waterBiopolymers, 89 (10) (2008), pp. 852-861Finding PDF…CrossrefView in ScopusGoogle Scholar
- Zheng, Lu et al., 2017X. Zheng, F. Lu, X. Xu, L. ZhangExtended chain conformation of β-glucan and its effect on antitumor activityJournal of Materials Chemistry B, 5 (28) (2017), pp. 5623-5631View in ScopusGoogle Scholar
- Zheng, Zhou et al., 2017X. Zheng, F. Zhou, X. Xu, L. ZhangUptake of intraperitoneally administrated triple helical β-glucan for antitumor activity in murine tumor modelsJournal of Materials Chemistry B, 5 (47) (2017), pp. 9337-9345View in ScopusGoogle Scholar
References