Abstract
β-(1→3)-D-glucans with β-(1→6)-glycosidic linked branches are known to be immune activation agents and are incorporated in anti-cancer drugs and health-promoting supplements. β-Glucan concentration was 9.2 g/L in a 200-L pilot scale fermentor using mutant strain Aureobasidium pullulans M-2 from an imperfect fungal strain belonging to A. pullulans M-1. The culture broth of A. pullulans M-2 had a faint yellow color, whereas that of the wild-type had an intense dark green color caused by the accumulation of melanin-like pigments. β-Glucan produced by A. pullulans M-2 was identified as a polysaccharide of D-glucose monomers linked by β-(1→3, 1→6)-glycosidic bonds through GC/MS and NMR analysis. When a conventional medium was used in the culture of A. pullulans M-2 in a 3-L jar fermentor, β-glucan concentration was 1.4-fold that produced by the wild-type. However, when a medium optimized by statistical experimental design was used with dissolved oxygen at 10%, the β-glucan concentration was 9.9 g/L with a yield of 0.52 (g β-glucan/g consumed sucrose), 2.9-fold that of the wild-type. This level of productivity was reproduced when the fermentation was scaled up 200-L. The industrial production of high β-glucan without melanin-like pigments is highly expected, as a health-promoting supplement or functional food.
Article PDF
https://link.springer.com/content/pdf/10.1007/s12257-013-0516-9.pdf
Similar content being viewed by others

Improved production of β-glucan by a T-DNA–based mutant of Aureobasidium pullulans
Article 27 August 2021
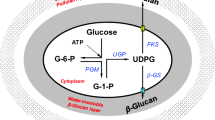
Enhanced β-glucan and pullulan production by Aureobasidium pullulans with zinc sulfate supplementation
Article 23 December 2019

Botryosphaeran – A Fungal Exopolysaccharide of the (1→3)(1→6)-β-D-Glucan Kind: Structure and Biological Functions
Chapter © 2019
Explore related subjects
Discover the latest articles, books and news in related subjects, suggested using machine learning.
- Aspergillus nidulans
- Fungal physiology
- Fungi
- Fungal genetics
- Polysaccharide sequencing
- Saccharomyces cerevisiae
References
- Takimoto, H., D. Wakita, K. Kawaguchi, and Y. Kumazawa (2004) Potentiation of cytotoxic activity in naïve and tumor-bearing mice by oral administration of hot-water extracts from Agaricus brazei fruiting bodies. Biol. Pharm. Bull. 27: 404–406.Article CAS Google Scholar
- Zhang, J., G. Wang, H. Li, C. Zhuang, T. Mizuno, H. Ito, C. Suzuki, H. Okamoto, and J. Li (1994) Antitumor polysaccharides from a Chinese mushroom, “Yuhuangmo,” the fruiting body of Pleurotus citrinopileatus. Biosci. Biotechnol. Biochem. 58: 1195–1201.Article CAS Google Scholar
- Delaney, B., R. J. Nicolosi, T. A. Wilson, T. Carlson, S. Frazer, G. -H. Zheng, R. Hess, K. Ostergren, J. Haworth, and N. Knutson (2003) Beta-glucan fractions from barley and oats are similarly antiatherogenic in hypercholesterolemic Syrian golden hamsters. J. Nutr. 133: 468–475.Google Scholar
- Izydorczyk, M. S. and J. E. Dexter (2008) Barley glucans and arabinoxylans: Molecular structure, physicochemical properties, and uses in food products. Food Res. Int’l. 41: 850–868.Article CAS Google Scholar
- Salar, R. K., M. Certik, and V. Brezova (2012) Modulation of phenolic content and antioxidant activity of maize by solid state fermentation with Thamnidium elegans CCF 1456. Biotechnol. Bioproc. Eng. 17: 109–116.Article CAS Google Scholar
- Kwiatkowski, S., U. Thielen, P. Glenney, and C. Moran (2009) A Study of Saccharomyces cerevisiae cell wall glucans. J. Inst. Brew. 115: 151–158.Article CAS Google Scholar
- Goodrige, H. S., C. N. Reyes, C. A. Becker, T. R. Katsumoto, J. Ma, A. J. Wolf, N. Bose, A. S. H. Chan, A. S. Magee, M. E. Danielson, A. Weiss, J. P. Vasilakos, and D. M. Underhill (2011) Activation of the innate immune receptor Dectin-1 upon formation of a ‘phagocytic synapse’. Nature 472: 471–475.Article Google Scholar
- Brown, G. D. and S. Gordon (2003) Fungal β-glucans and mammalian immunity. Immunity 19: 311–315.Article CAS Google Scholar
- Lull, C., H. J. Wichers, and H. F. J. Savelkoul (2005) Antiinflammatory and immunomodulating properties of fungal metabolites. Mediat. Inflamm. 2: 63–80.Article Google Scholar
- Thompson, I. J., P. C. F. Oyston, and D. E. Williamson (2010) Potential of the β-glucans to enhance innate resistance to biological agents. Expert Rev. Anti. Infect. Ther. 8: 339–352.Article CAS Google Scholar
- Hong, F., J. Yan, J. T. Baran, D. J. Allendorf, R. D. Hansen, G. R. Ostroff, P. X. Xing, N. -K. V. Cheung, and G. D. Ross (2006) Mechanism by which orally administered β-1,3-glucans enhance the tumoricidal activity of antitumor monoclonal antibodies in murine tumor models. J. Immunol. 173: 797–806.Google Scholar
- Li, B., D. J. Allendorf, R. Hansen, J. Marroquin, D. E. Cramer, C. L. Harris, and J. Yan (2007) Combined yeast β-glucan and antitumor monoclonal antibody therapy requires C5a-mediated neutrophil chemotaxis via regulation of decay-accelerating factor CD55. Cancer Res. 67: 7421–7430.Article CAS Google Scholar
- Yatawara, L., S. Wickramasinghe, M. Nagataki, M. Takamoto, H. Nomura, Y. Ikeue, Y. Watanabe, and T. Agatsuma (2009) Aureobasidium-derived soluble branched (1,3–1,6) β-glucan (Sophy β-glucan) enhances natural killer activity in Leishmania amazonensis-infected mice. Kor. J. Parasitol. 47: 345–351.Article CAS Google Scholar
- Muramatsu, D., A. Iwai, S. Aoki, H. Uchiyama, K. Kawata, Y. Nakayama, Y. Nikawa, K. Kusano, M. Okabe, and T. Miyazaki (2012) β-Glucan derived from Aureobasidium pullulans is effective for the prevention of influenza in mice. PLoS ONE 7: e41399.Article CAS Google Scholar
- Miyawaki, K., K. Terao, S. Yamakita, S. Takahashi, Y. Ikeue, N. Fujii, T. Onaka, H. Muramatsu, and S. Nagata (2010) Relationship between the functional β-glucan polysaccharide-production and the cell morphologies of Aureobasidium pullulans. Seibutsukogaku 88: 634–641.CAS Google Scholar
- Hamada, N., K. Deguchi, T. Ohmoto, K. Sakai, T. Ohe, and H. Yoshizumi (2000) Ascorbic acid stimulation of production of a highly branched β-glucan by Aureobasidium pullulans K-1-oxalic acid, a metabolite of ascorbic acid as the stimulating substance. Biosci. Biotechnol. Biochem. 64: 1801–1806.Article CAS Google Scholar
- Wei, N. -W. V., C. C. Wallace, C. -F. Dai, K. R. M. Pillay, and C. A. (2006) Chen Analyses of the ribosomal internal transcribed spacers (ITS) and the 5.8S gene indicate that extremely high rDNA heterogeneity is a unique feature in the Scleractinian coral genus Acropora (Scleractinia; Acroporidae). Zool. Stud. 45: 404–418.CAS Google Scholar
- Virtudazo, E. V., H. Nakamura, and M. Kakishima (2001) Phylogenic analysis of sugarcane rusts based on sequence of ITS, 5.8 S rRNA and D1/D2 regions of LSU rRNA. J. Gen. Plant Pathol. 67: 28–36.Article CAS Google Scholar
- Anumula, K. R. and P. B. Taylor (1992) A comprehensive procedure for preparation of partially methylated alditol acetates from glycoprotein carbohydrates. Anal. Biochem. 203: 101–108.Article CAS Google Scholar
- Schmid, F., B. A. Stone, B. M. McDougall, A. Bacic, K. L. Martin, R. T. C. Brownlee, E. Chai, and R. J. Seviour (2001) Structure of epiglucan a highly side-chain/branched (1→3;1→6)-β-glucan from the micro fungus Epicoccum nigrum Ehrenb. ex. Schlecht. Carbohydr. Res. 331: 163–171.Article CAS Google Scholar
- Box, G. E. P. and D. W. Behnken (1960) Some new three level designs for the study of quantitative variables. Technometric 2: 455–475.Article Google Scholar
- Lopez, J. C., J. S. Perez, J. M. F. Sevilla, F. G. A. Fernandez, E. M. Grima, and Y. Chisti (2004) Fermentation optimization for the production of lovastatin by Aspergillus terreus: Use of response surface methodology. J. Chem. Technol. Biotechnol. 79: 1196–1126.Google Scholar
- EI-Refai, H. A., E. R. EI-Helow, M. A. Amin, L. A. Sallam, and H. -A. A. Salem (2010) Application of multi-factorial experimental designs for optimization of biotin production by a Rhizopus nigricans strain. J. Am. Sci. 6: 179–187.Google Scholar
Author information
Authors and Affiliations
- Tokyo Head Office, Aureo Co. Ltd., Tokyo, 108-0071, JapanNaoyuki Moriya, Yukiko Moriya, Kisato Kusano & Yukoh Asada
- Kazusa Factory, Aureo Co. Ltd., Kimitsu-shi, 292-1149, JapanHideo Nomura, Yukoh Asada, Hirofumi Uchiyama & Mitsuyasu Okabe
- Laboratory of Biotechnology, Graduate School of Green Science and Technology, Shizuoka University, Shizuoka, 422-8529, JapanHirofumi Uchiyama & Enoch Y. Park
References
