Fungal β-Glucans and Mammalian Immunity
β-Glucans are structural cell wall polymers of many fungi which possess immunomodulatory activities. Although the therapeutic benefits associated with these compounds, particularly as anti-infective and antitumorigenic agents, have led to a large body of published research over the last five decades, it is still unclear how these carbohydrates mediate their effects. Recent studies, however, are starting to shed some light on the cellular receptors and molecular mechanisms involved, which also have direct relevance on the innate immune response to fungal pathogens.
Main Text
Over the last few years, there has been a resurgence of interest in innate immunity and the mechanisms utilized by this evolutionarily ancient arm of the immune system to distinguish self from nonself. What has become apparent from these studies is that the innate recognition of nonself is mediated largely through germline-encoded molecules that recognize conserved microbial structures, the so-called “pattern recognition receptors” (Janeway, 1992). The recent discovery of the Toll-like receptors has also provided insights into how the inflammatory signals are generated in response to microbes. Despite all these discoveries, however, little is known about the innate recognition of fungal pathogens or their components, even though fungal infections are becoming increasingly frequent in immunocompromised individuals worldwide and represent over 10% of all nosocomial infections (van Burik and Magee, 2001). Here we will review the innate recognition and response to one group of conserved fungal components, the β-glucans, and highlight recent findings which have relevance both in terms of the immunomodulatory activities of these carbohydrates and their importance in immunity to fungal pathogens.
β-Glucans are a heterogeneous group of glucose polymers, consisting of a backbone of β(1→3)-linked β-D-glucopyranosyl units with β(1→6) linked side chains of varying distribution and length. These polysaccharides are major cell wall structural components in fungi and are also found in plants and some bacteria. As they are not found in animals, these carbohydrates are considered to be classic pathogen-associated molecular patterns (Janeway, 1992) and are recognized by the innate immune system of vertebrates, as well as invertebrates.
Surprisingly, the recognition of β-glucans by vertebrates differs significantly from that of invertebrates. As we shall see, vertebrate recognition of soluble β-glucans appears to occur exclusively via a number of cell surface receptors, and although complement opsonization does contribute to the recognition of particulate glucans, no plasma molecules recognizing this carbohydrate structure have been identified. In contrast, the recognition of β-glucans appears to occur primarily in the hemolymph of invertebrates via a number of completely unrelated proteins, such as horseshoe crab factor G and gram-negative binding proteins (GNBPs). Only one cellular receptor has been shown to recognize β-glucans in invertebrates, the Drosophila scavenger receptor (dSRCI). Despite these differences, the recognition of β-glucans in both systems results in the triggering of immune responses, designed primarily for the control of fungal pathogens. The invertebrate recognition and response to these carbohydrates is reviewed elsewhere (Muta and Iwanaga, 1996).
Historical Interest in β-Glucans
The healing properties of mushrooms have been known for thousands of years, with the first recorded report of their medicinal qualities dating from around 3000 BC, and although a number of fungal components have been implicated in these properties, β-glucans have attracted the most attention. The interest in these polymers originated in the early 1900s, when the ability of yeast to inactivate serum complement was first described. This led to the development of an insoluble yeast cell wall particle, termed zymosan, which was subsequently used to help define the pathway of alternative complement activation. Later studies showed that the direct intravenous injection of zymosan could activate the immune system, stimulating protective host responses (see below). Although these particles were found to consist of a variety of components (including glucans, mannans, chitin, protein, and lipids), β-glucan was identified as the biologically active constituent (see Fitzpatrick and DiCarlo [1964] for a more detailed review). Since the discovery of its stimulatory activities, zymosan has been a particle of choice for many studies of immune function both in vivo and in vitro, including inflammation, phagocytosis, arachidonate release, and cell migration.
Biological Effects of β-Glucans
There is now a considerable body of published research detailing the biological effects of β-glucans but, unfortunately, the literature is inconsistent and often contradictory. This has mainly been due to the use of β-glucans with different molecular weights (MW) and chemical modifications, derived from a variety of fungal sources, including many common fungi and yeasts (Table 1). It is known that the immunomodulatory effects of β-glucans are influenced by their degree of branching, polymer lengths, and tertiary structure, but there is still no consensus on the basic structural requirements for biological activity. Although this review will focus primarily on fungal β-glucans, β-glucans from other sources appear to have similar properties.
| β-Glucan | Source (common name) |
| zymosan | Saccharomyces cerevisiae |
| glucan phosphate | Saccharomyces cerevisiae |
| PGG-Glucan | Saccharomyces cerevisiae |
| lentinan | Lentinus edodes (Shiitake mushroom) |
| schizophyllan | Schizophyllum commune |
| scleroglucan | Sclerotium glucanicum |
| grifolan | Grifola frondosa (Maitake mushrooms) |
| SSG-Glucan | Sclerotinia sclerotiorum |
Commonly Used Biologically Active β-Glucans and Their Fungal Sources
In general, in vitro studies have suggested that large MW or particulate β-glucans (such as zymosan) can directly activate leukocytes, stimulating their phagocytic, cytotoxic, and antimicrobial activities, including the production of reactive oxygen and nitrogen intermediates. In addition, these carbohydrates stimulate the production of proinflammatory mediators, cytokines, and chemokines, such as IL-8, IL-1β, IL-6, and TNF-α (Czop, 1986; Williams et al., 1996). Stimulation by particulate β-glucans also enhances the ability of macrophages to recognize and clear apoptotic cells, through upregulation of the PS receptor (Fadok et al., 2000).
Intermediate or low MW β-glucans (such as glucan phosphate) possess biological activity in vivo, but their cellular effects are less clear. In some studies, these glucans have been shown to activate leukocytes in vitro, priming the cells for enhanced responses to a secondary challenge, with, for example, LPS or intact bacteria. This is thought to be mediated, at least in part, by the activation of nuclear transcription factors, including NFκB and NF-IL-6 (Adams et al., 1997; Battle et al., 1998). Although these glucans do not induce cytokines, such as TNF-α and IL-1β, there are some reports in which stimulation of IL-8 and IL-6 production occurred. In other studies, however, β-glucans have been shown to suppress proinflammatory cytokine production in response to a secondary challenge (Nakagawa et al., 2003). Understanding the molecular mechanisms behind the cellular effects of these β-glucans will help to solve these apparent contradictions. Very short β-glucans (<5000–10,000 MW; such as laminarin) are generally considered inactive (see Bohn and BeMiller [1995] for a more detailed review on the structure-function relationships of β-glucans).
The in vivo administration of β-glucans has been shown to potentiate host responses against a variety of conditions, including tumor development and infection with fungal, bacterial, viral, and protozoal pathogens (Ross et al., 1999; Tzianabos, 2000). This has led to a number of clinical trials using β-glucans in tumor immunotherapy and as prophylactic agents for the prevention of infections in surgical patients, with promising results. Although there has not yet been widespread pharmaceutical development of these compounds, they are being used for tumor immunotherapy in Japan. The published evidence of the beneficial effects of β-glucans has also led to a growing number of alternative medicines based on these polymers.
Perhaps another contributing factor to the biological activities of β-glucans is their longevity in mammalian systems. Vertebrate cells do not possess (1→3)β-glucanases and cannot rapidly degrade these carbohydrates, metabolizing them slowly through oxidation (Nono et al., 1991). In vivo, the clearance of β-glucans depends on their MW, with low MW glucans being secreted through glomerular filtration and larger glucans being retained primarily in the liver and degraded by Kupffer cells, a process which may take several weeks (Suda et al., 1996).
Despite their beneficial uses, the stimulatory activities of β-glucans can also have detrimental effects on the host. Particulate β-glucans, for example, induce granuloma formation after systemic administration, although the development of soluble, biologically active, intermediate MW β-glucans has overcome this side effect (Williams et al., 1996). In addition, these polymers can induce lethal toxicity in mice, when combined with nonsteroidal anti-inflammatory drugs (Takahashi et al., 2001), and may be involved in asthma, as a result of airway inflammation after inhalation (Rylander and Lin, 2000).
Leukocyte Receptors for β-Glucans
Evidence for cellular β-glucan receptors first came from the demonstration that nonopsonic zymosan recognition by human monocytes was β-glucan dependent (Czop, 1986). It should be noted, however, that some leukocyte populations also express other nonopsonic receptors for zymosan, such as the mannose binding lectins (Taylor et al., 2002). β-Glucan receptor activity has subsequently been reported on a variety of other leukocytes, including macrophages, neutrophils, eosinophils, and NK cells, as well as on nonimmune cells including endothelial cells, alveolar epithelial cells, and fibroblasts. Nonopsonic recognition of β-glucans by these cells has been ascribed to multiple receptors (Battle et al., 1998), and indeed a number of β-glucan receptors have been identified, including CR3, lactosylceramide, scavenger receptors, and Dectin-1. Of these receptors, however, only Dectin-1 has been clearly shown to have a role in mediating the biological response to β-glucans (see below).
CR3
CR3 is a heterodimeric integrin receptor, consisting of the αm (CD11b) and β2 (CD18) chains and expressed on myeloid cells, NK cells, and selected lymphocytes. CR3 functions as an adhesion molecule, through recognition of endothelial ICAM-1, and as a phagocytic receptor for iC3b-opsonized particles, including opsonized particulate glucans. CR3 also possesses a lectin domain which maps to a site C-terminal to the I-domain, and recognizes selected monosaccharides and a variety of β-glucans, including zymosan, although the highest-affinity polymeric ligand contained very little β-glucan and consisted mostly of mannose. The presence of this domain was first noted using inhibitory monoclonal antibodies and cells from patients with β2-integrin deficiencies (Ross et al., 1999). Although the CR3-dependent cytotoxicity of iC3b-coated tumor cells is dependent on β-glucan priming of the leukocyte, the exact role of CR3 in the mediation of the response to β-glucans is unclear. Purified CR3 does not interact with these carbohydrates, and leukocytes lacking CR3 can still bind and respond normally to β-glucans (Brown et al., 2002; Czop and Kay, 1991; Muller et al., 1996; Van Strijp et al., 1993).
Lactosylceramide
Lactosylceramide (LacCer; CDw17) is a glycosphingolipid found in the plasma membranes of many cells and was identified as a β-glucan receptor from biochemical analyses of the interactions between PGG-glucan and isolated human leukocyte membrane components (Zimmerman et al., 1998). It has been suggested that the interaction of β-glucan with this receptor can induce macrophage inflammatory protein (MIP)-2 and the activation of NFκB and can enhance the neutrophil oxidative burst and antimicrobial functions, but the mechanisms behind these activities are unknown.
Scavenger Receptors
Although no specific receptor has been identified, a number of studies suggest that macrophage scavenger receptors can recognize β-glucans. The biochemical demonstration that soluble β-glucans could inhibit the interactions of monocyte membranes with classic scavenger ligands is perhaps the best supporting evidence, but may be attributed to the charge of the glucans tested (Rice et al., 2002).
Dectin-1
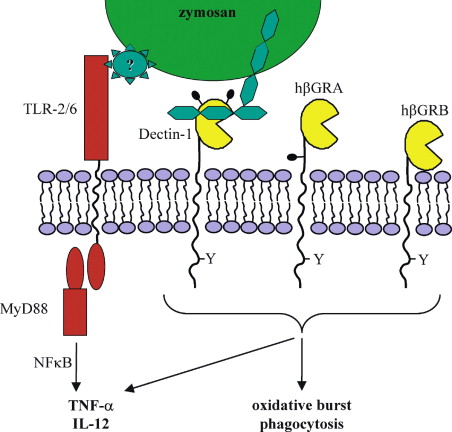
Originally identified as a receptor that recognized an unidentified ligand on T lymphocytes, promoting their cellular proliferation (Ariizumi et al., 2000), Dectin-1 was reidentified as a β-glucan receptor from a macrophage cDNA expression library screened with zymosan (Brown and Gordon, 2001). The receptor possesses a single nonclassical C-type carbohydrate recognition domain (CRD) connected to the transmembrane region by a stalk, and a cytoplasmic tail possessing an immunoreceptor tyrosine-based activation (ITAM) motif (Figure 1). Human Dectin-1 differs from its murine counterpart in that it is alternatively spliced, in a cell-specific manner, giving rise to several isoforms of which only two are functional (Willment et al., 2001). Dectin-1 recognizes carbohydrates containing β-1,3 and/or β-1,6 glucan linkages, and is expressed on cells of the monocyte/macrophage and neutrophil lineages, and at lower levels on dendritic cells and a subpopulation of splenic T cells (Brown and Gordon, 2001; Taylor et al., 2002). Dectin-1 was shown to be the major receptor for both soluble and particulate β-glucans on macrophages, and contributed to the recognition of opsonized glucan particles (Brown et al., 2002). More importantly, Dectin-1 was recently demonstrated to mediate cellular responses to particulate β-glucans, including the production of proinflammatory cytokines (Brown et al., 2003; see below).
Molecular Mechanisms of β-Glucan-Induced Leukocyte Activation
The immunomodulatory activities of β-glucans are still far from being understood, particularly those of the intermediate MW glucans, but recent studies have started to shed some light on the mechanisms behind the proinflammatory response induced by large MW and particulate β-glucans. In particular, following the demonstration that Toll-like receptors (TLR) have a central role in signaling the induction of inflammatory reactions, TLR-2 was shown to be critically required in the response to zymosan (Underhill et al., 1999). TLR-2, which can interact with this particle directly (Sato et al., 2003), forms functional pairs with TLR-6 to induce cytokine and chemokine production (Ozinsky et al., 2000). In addition, downstream components of the TLR signaling pathway are required, including MyD88 and NFκB (Kataoka et al., 2002; Young et al., 2001).
More recently, the proinflammatory response to zymosan was shown to also require a signal from Dectin-1 (Figure 1). Using transfected macrophages and HEK293 cells, it was demonstrated that for NFκB activation and the production of TNF-α and IL-12, signals from both Dectin-1 and TLR-2 were needed (Brown et al., 2003; Gantner et al., 2003). Although the mechanism is unknown, the generation of this response occurred at the cell surface and required a functional ITAM in cytoplasmic tail of Dectin-1. The ITAM motif became phosphorylated after zymosan binding, suggesting that signaling may occur in a fashion similar to that of other ITAM-containing receptors, such as the Fc receptor.
In addition, Dectin-1 can trigger cellular responses to β-glucans independently of the TLRs, including phagocytosis and the oxidative burst (Brown and Gordon, 2001; Gantner et al., 2003). These activities are also mediated by signaling events triggered from the cytoplasmic tail. TLR-2, although recognizing some component of zymosan (Gantner et al., 2003), does not recognize β-glucans, and thus Dectin-1 signaling may play a central role in immunomodulation mediated by these carbohydrates. In addition, this suggests that the strong proinflammatory activities reported for some β-glucans may be due to unidentified TLR2-triggering contaminants. Characterization of the Dectin-1 knockout mouse, when available, should clarify these issues. In a broader context, these results represent a new paradigm in innate immunity, demonstrating for the first time that the response to microbes, or their components, can require signals from a specific receptor, in addition to those mediated by the TLRs.
Role of β-Glucans in Fungal Pathogenesis
The innate immune response is essential for the control of fungal infections, and there is increasing evidence that β-glucans are involved in initiating many aspects of this response. The recognition of fungal pathogens occurs through both opsonic (mainly complement) and nonopsonic mechanisms, and as conserved structural components, β-glucans, along with mannans and other cell wall components, play an important role in the nonopsonic recognition of these pathogens. Indeed, many of the β-glucan receptors, including CR3, lactosylceramide, and Dectin-1, have been shown to contribute to the recognition and phagocytosis of these organisms.
As we have already seen, β-glucans, especially in particulate form, can induce proinflammatory and antimicrobial responses through the TLRs and Dectin-1. Many of these responses are required for the control of fungal infections, such as the production of TNF-α, which is induced by Dectin-1 in response to live fungi and is an essential early cytokine required for the control of infections with C. albicans, A. fumigatus, C. neoformans, and H. capsulatum (Herring and Huffnagle, 2002). This is also true for IL-12, another important antifungal cytokine required for the induction of IFN-γ and the polarization toward a protective Th1 type adaptive response (Romani, 2000). Thus β-glucans appear to have an important role in the innate immune response to fungal pathogens and in initiating a protective adaptive response.
Patients who suffer from systemic fungal infections, including those caused by candida, aspergillus, and cryptococcus species, have been described to possess high levels of circulating β-glucans in their plasma (Obayashi et al., 1995). Although the characteristics of these carbohydrates and their effects during infection are unknown, it is possible that they may have modulating effects on the immune system. These circulating β-glucans have been proposed to be a diagnostic indicator of mycotic infections, and at least one detection kit is commercially available.
Finally, given the response of the immune system to fungal β-glucans, it is tempting to speculate that pathogens may avoid immune recognition by masking their β-glucan. Indeed, some pathogens, such as Cryptococcus neoformans, avoid recognition through the production of an extracellular capsule (Kozel, 1995). It is tempting to speculate that organisms also avoid immune recognition by changing their cell wall composition during infection, such as occurs with Paracoccidioides brasiliensis, which displays a transition from β-glucan to α-glucan in the cell wall upon infection of the lung (Borges-Walmsley et al., 2002).
Conclusions
In the defense against fungal pathogens, mammals have evolved mechanisms to recognize and respond to their conserved structural components, particularly β-glucans. Our understanding of these mechanisms has not only provided insights into the workings of the innate immune system but will allow the future exploitation of the therapeutic potential of these carbohydrates.
Acknowledgements
We thank the Wellcome Trust and Medical Research Council for financial support.
References
Herring, A.C., and Huffnagle, G.B. (2002). In Immunology of Infectious Diseases, S.H.E. Kaufmann, ed. (Washington, DC: ASM Press).
References
DOI: 10.1016/S1074-7613(03)00233-4

00233-4/asset/532ef956-27e3-4654-a83e-9ce0e4fe64b4/main.assets/gr1.jpg)