bstract
Detachment of adherent epithelial cells from the extracellular matrix induces apoptosis, a process known as anoikis. We have shown that DAP3 is critical for anoikis induction. However, the mechanism for anoikis induction mediated by DAP3 is still unclear. Here, we show that interferon-β promoter stimulator 1 (IPS-1) binds DAP3 and induces anoikis by caspase activation. Recently, IPS-1 has been shown to be critical for antiviral immune responses, although there has been no report of its function in apoptosis induction. We show that overexpression of IPS-1 induces apoptosis by activation of caspase-3, -8, and -9. In addition, IPS-1 knockout mouse embryonic fibroblasts were shown to be resistant to anoikis. Interestingly, IPS-1 expression, recruitment of caspase-8 to IPS-1, and caspase-8 activation were induced after cell detachment. Furthermore, DAP3-mediated anoikis induction was inhibited by knockdown of IPS-1 expression. Therefore, we elucidated a novel function of IPS-1 for anoikis induction by caspase-8 activation.
Similar content being viewed by others
Caspase-9 driven murine model of selective cell apoptosis and efferocytosis
Article Open access24 January 2023

Caspases: structural and molecular mechanisms and functions in cell death, innate immunity, and disease
Article Open access05 May 2025
Apoptotic caspase inhibits innate immune signaling by cleaving NF-κBs in both Mammals and Flies
Article Open access24 August 2022
Main
Apoptosis, the programmed physiological cell death, is critical for modeling tissues and maintaining homeostasis in multicellular organisms.1 Two major pathways to induce apoptosis are known as the ‘extrinsic’ and ‘intrinsic’ pathways.2, 3 The extrinsic pathway is triggered by binding of tumor necrosis factor α (TNF-α) or Fas ligand to cell surface receptors. Binding of these ligands induce recruitment of the adaptor protein, TNF receptor 1-associated death domain protein or Fas-associated death domain (FADD) with procaspases to death receptors, leading to the procaspase activation and apoptosis induction. The intrinsic pathway, also referred to as the mitochondrial apoptosis pathway, responds to intracellular signals induced by DNA damage, cytokine deprivation, or various stimuli.4 Activation of this pathway triggers the release into the cytoplasm of proteins that mediate cell death, such as cytochrome c.5 Recently, it is shown that multiple pathways activated by intracellular stress signals converge at the mitochondrion to induce loss of outer membrane integrity in a process regulated by Bcl-2 family members.6 The extrinsic pathway has also been reported to potentiate the mitochondrial pathway by triggering cytochrome c release through caspase-8-cleaved Bid protein, a pro-apoptotic member of the Bcl-2 family that translocates to the mitochondria.7, 8 Recently, DAP3 is shown to be essential for mitochondrial homeostasis and contributing to the extrinsic pathway for apoptosis induction.9
Adhesion to an appropriate extracellular matrix is essential for the survival of many normal cells, and loss of adhesion induces programmed cell death, a phenomenon known as anoikis. Anoikis was first observed in endothelial and epithelial cells, in which loss of matrix attachment, even in the presence of serum, induces cell death. Expression of certain oncogenes can provide protection from anoikis,10, 11, 12, 13 leading to metastasis induction. We have shown that death-associated protein 3 (DAP3) is critical for anoikis induction through interaction with FADD, correlating with caspase-8 activation.14 Downregulation of DAP3 expression inhibited anoikis and overexpression of DAP3 augmented cell death through caspase activation after cell detachment. Furthermore, association of DAP3 with FADD and caspase-8 activation were induced by cell detachment. Interferon-β promoter stimulator 1 (IPS-1), also known as mitochondrial antiviral signaling protein, virus-induced signaling adaptor, and caspase recruitment domain (CARD) adaptor inducing IFN-β (Cardif), was recently identified as an adaptor linking RIG-I and Mda5 to the downstream signaling molecules.15, 16, 17, 18, 19 IPS-1 contains the CARD-like domain that is responsible for the interaction with RIG-I or Mda5. In addition, IPS-1 contains a transmembrane region that targets this protein to the mitochondrial outer membrane.19 The mitochondrial localization of IPS-1 is essential for triggering downstream signals, including FADD and caspase-8.19, 20 However, the functional role of IPS-1 in apoptosis or anoikis induction is not reported.
Here, we report that IPS-1 is associated with DAP3 and that IPS-1 expression is induced after cell detachment. Overexpression of IPS-1 induced apoptosis and the cleavage of caspase-3, -8, and -9. In addition, IPS-1 knockout (KO) mouse embryonic fibroblasts (MEFs) were resistant to anoikis and activation of caspase-3, -8, and -9 after cell detachment was reduced in these cells. Conversely, apoptosis induction by death receptor stimulation was not inhibited in IPS-1 KO MEFs. Furthermore, caspase-8 was recruited to IPS-1 and activated after cell detachment. Therefore, we have elucidated a novel function of IPS-1 to activate caspase-8 for anoikis induction.
Results
IPS-1 is associated with DAP3 and induces caspase-dependent apoptosis
To analyze the downstream signal of DAP3, we screened its binding proteins and identified IPS-1 to bind it (Figure 1a; Supplementary Figure 1). Expression of IPS-1 by transfection of its expression vector induced morphological change in the cell shape, detachment, and cell death in 293T cells (Figure 1b). In addition, DNA fragmentation (Figure 1c) and caspase-3, -8, or -9 activation (Figure 1d) were detected by the expression of IPS-1. Next, we clarified the critical region of IPS-1 to induce apoptosis using expression vector plasmids for deletion mutants of IPS-1, such as IPS-1N (the N-terminal CARD), IPS-1C (the C-terminal non-CARD region), and IPS-1ΔMito (deletion of mitochondrial membrane region) (Figure 1e; Supplementary Figure 2). Expression of IPS-1C but not IPS-1N and IPS-1ΔMito induced apoptosis, indicating that the mitochondrial membrane region of IPS-1 is critical for apoptosis induction (Figure 1e).
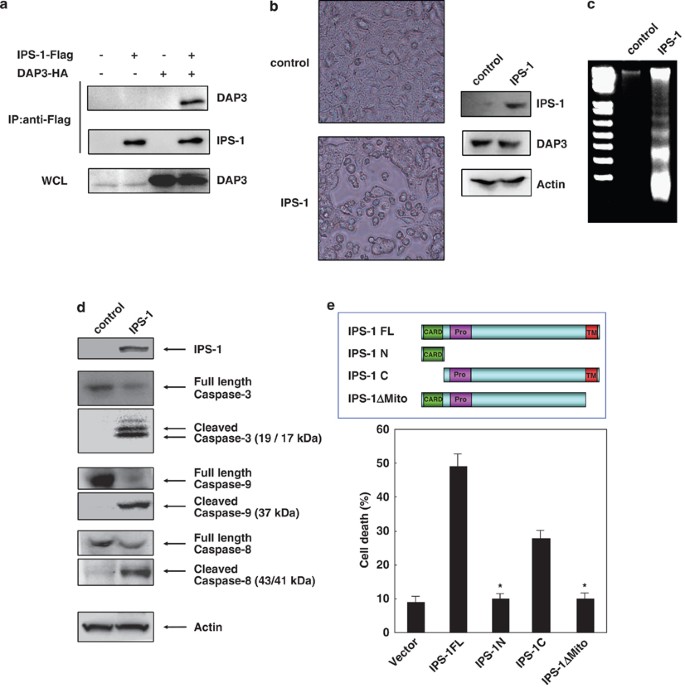
IPS-1 KO MEF cells are resistant to anoikis by reduced activation of caspase-3, -8, and -9 after cell detachment
We investigated whether IPS-1 is involved in apoptosis signals mediated by death receptors. After IPS-1 KO21 (Figure 2a) and WT (wild type) MEFs were stimulated with TNF-α, TRAIL, or anti-Fas Ab in combination with cycloheximide, cells were analyzed for apoptosis induction. No significant difference for caspase activation or apoptosis induction was observed by the stimulation of these death receptors between IPS-1 KO and WT MEFs (Supplementary Figure 3).
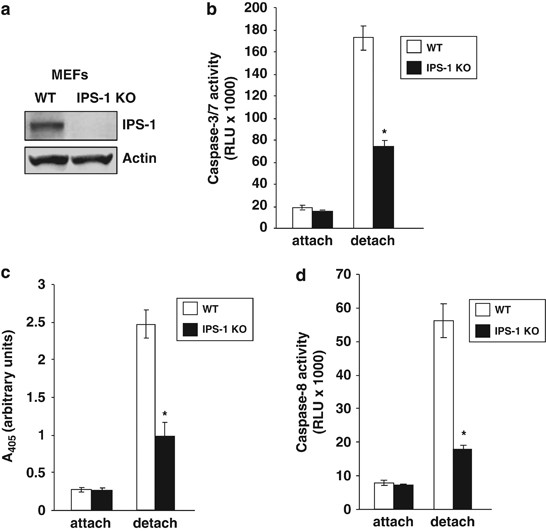
Next, we investigated whether IPS-1 is involved in anoikis induction. At 24 h after IPS-1 KO or WT MEF cells were detached and cultured in suspension, caspase-3/7 activity in these cells was analyzed. Activity of caspase-3/7 induced by cell detachment in IPS-1 KO MEFs was lower than that in WT MEFs (Figure 2b). Furthermore, anoikis induction was inhibited in IPS-1 KO MEFs in comparison with WT MEFs at 24 h after cell detachment analyzed by DNA fragmentation ELISA assay (Figure 2c). In addition, we examined caspase-8 activity in IPS-1 KO and WT MEFs before and after cell detachment. It was shown that caspase-8 activity was lower in IPS-1 KO MEFs than in WT MEFs after cell detachment (Figure 2d). In the same experiment, caspase-9 activity was also lower in IPS-1 KO MEFs than in WT MEFs (Supplementary Figure 4).
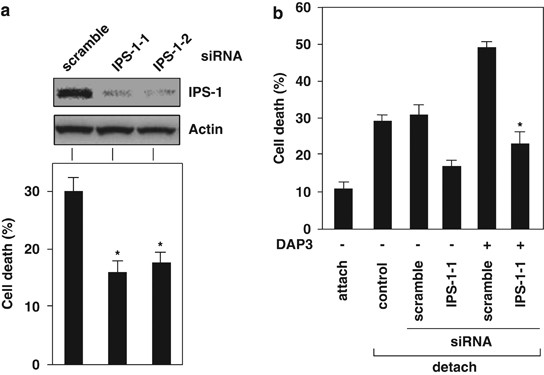
DAP3-mediated anoikis induction was inhibited by knockdown of IPS-1 expression
We examined the functional relationship of IPS-1 with DAP3 for anoikis induction. Knockdown of IPS-1 expression by siRNA transfection inhibited anoikis induction (Figure 3a; Supplementary Figure 5a and b). DAP3-mediated anoikis induction was suppressed by knockdown of IPS-1 expression in 293T cells (Figure 3b; Supplementary Figure 5c). Conversely, we examined whether IPS-1-mediated anoikis induction is inhibited by the knockdown of DAP3 expression9 for anoikis induction. It was shown that IPS-1-induced apoptosis was not suppressed by the knockdown of DAP3 expression (Supplementary Figure 6). In addition, mutant of DAP3, DAP3(T237E) could be associated with IPS-1 (Supplementary Figure 7).
IPS-1 interacts with caspase-8 and induces its activation after cell detachment
We examined whether caspase-8 interacts with IPS-1 using 293T cells. We have shown that caspase-8 associated with IPS-1 and the mitochondrial membrane region of IPS-1 was necessary for its association (Figure 4a). Furthermore, we performed immunoprecipitation experiments for the analysis of the interaction of IPS-1 with FADD and caspase-8.
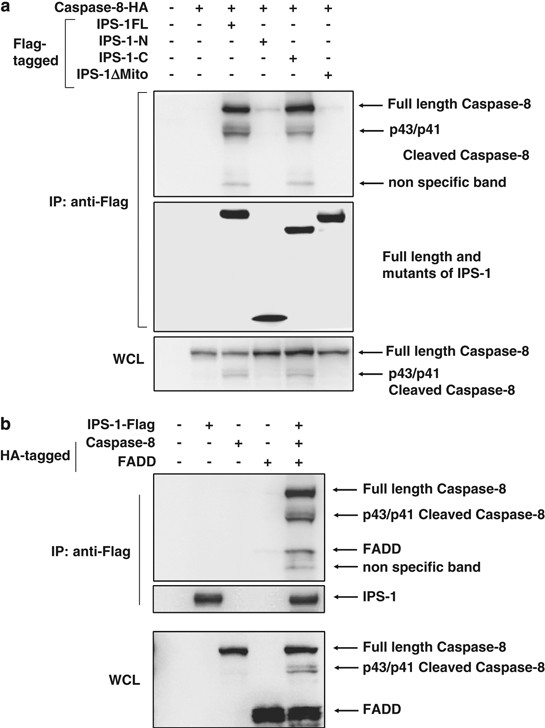
Both FADD and caspase-8 associated with IPS-1, and casapse-8 strongly associated with IPS-1 as compared with FADD (Figure 4b).
IPS-1 is upregulated after cell detachment and induces anoikis
To determine whether expression of IPS-1 is regulated during anoikis induction, we analyzed the expression of IPS-1 in MCF-10A cells, in which apoptosis was induced at both 48 and 72 h after cell detachment. IPS-1 expression was induced and reached to maximal level at 48 h after detachment (Figure 5a).
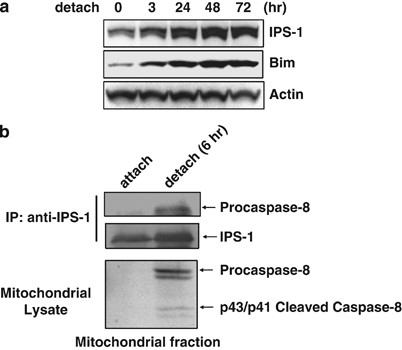
We next examined whether caspase-8 is recruited to IPS-1 in the mitochondria after cell detachment. We found that caspase-8 was co-immunoprecipitated with IPS-1 in a mitochondrial fraction after cell detachment (Figure 5b).
Discussion
Recent studies suggest that DAP3, a pro-apoptotic protein, is required for mitochondrial homeostasis in vivo and contributes to the extrinsic pathway for apoptosis.9 It has been shown that DAP3 is important for apoptosis induction.14, 22, 23 However, the mechanism for DAP3-mediated apoptosis induction is still unknown. Therefore, we screened its binding proteins and identified IPS-1 as a DAP3-binding protein (Figure 1a; Supplementary Figure 1). As shown in Figure 1b, expression of IPS-1 induced cell morphological change, detachment, and cell death. As DNA fragmentation (Figure 1c) and caspase-3, -8, or -9 activation (Figure 1d) were detected, it is suggested that IPS-1 expression induces apoptosis by caspase activation. Next, to identify the critical region of IPS-1 for apoptosis induction, we examined the function of some IPS-1 mutants. As expression of IPS-1C but not IPS-1N and IPS-1ΔMito induced apoptosis, it is suggested that the mitochondrial membrane region of IPS-1 is critical for apoptosis induction (Figure 1e; Supplementary Figure 2).
IPS-1 interacts with FADD, caspase-8, and -10.20 As shown in Figure 1d, we found that overexpression of IPS-1 cleaves caspase-3, -8, and -9. Therefore, we examined whether IPS-1 is critical for apoptosis signals mediated by death receptors. IPS-1 KO21 (Figure 2a) and WT MEFs were stimulated with TNF-α, TRAIL, or anti-Fas Ab in combination with cycloheximide but there was no significant difference for caspase activation or apoptosis induction (Supplementary Figure 3). These results show that IPS-1 is not critical for apoptosis induction mediated by death receptors.
We have shown earlier that DAP3 is critical for anoikis induction.14 Therefore, we next examined whether IPS-1 is critical for anoikis induction. After IPS-1 KO or WT MEF cells were detached and cultured in suspension, caspase-3/7 activity of IPS-1 KO MEFs was lower than that of WT MEFs (Figure 2b). It is shown that IPS-1 is involved in caspase-3/7 activation after cell detachment. Furthermore, anoikis induction was inhibited in IPS-1 KO MEFs in comparison with WT MEFs after cell detachment (Figure 2c). These results indicate that IPS-1 is critical for anoikis induction. Casapase-8 activation is an initiating event in anoikis induction.24, 25 Therefore, we examined caspase-8 activity and it was shown to be lower in IPS-1 KO MEFs than in WT MEFs after cell detachment (Figure 2d). As shown in Supplementary Figure 4, caspase-9 activity was also lower in IPS-1 KO MEFs than in WT MEFs. These results show that IPS-1 is critical for caspase-3, -8, and -9 activation after cell detachment.
Knockdown of IPS-1 expression by siRNA inhibited anoikis induction in 293T cells (Figure 3a; Supplementary Figure 5a and b). We have shown earlier that DAP3 was critical for anoikis induction,14 and we show here that DAP3 associates with IPS-1 (Figure 1a; Supplementary Figure 1). Therefore, we evaluated the functional relationship of IPS-1 with DAP3 for anoikis induction. DAP3-mediated anoikis induction was suppressed by knockdown of IPS-1 expression (Figure 3b; Supplementary Figure 5c). Conversely, we examined whether IPS-1-mediated anoikis induction is inhibited by the knockdown of DAP3 expression. IPS-1-induced apoptosis was shown not to be suppressed by the knockdown of DAP3 expression (Supplementary Figure 6). These results indicated that IPS-1 is located at the downstream of DAP3 in anoikis signal. DAP3(T237E), which is the phospho-mimic mutant,14 could be associated with IPS-1 (Supplementary Figure 7), the phosphorylation of DAP3 by Akt is not involved in the interaction of DAP3 with IPS-1.
It was shown that IPS-1 interacts with FADD,16 and that overexpression of IPS-1 induced apoptosis (Figure 1b and c) and cleavage of caspase-8 (Figure 1d). Therefore, we examined the interaction of caspase-8 with IPS-1 and caspase-8 was shown to be associated with IPS-1 (Figure 4a). In addition, the mitochondrial membrane region of IPS-1 was shown to be essential for its interaction. Furthermore, we examined the interaction of IPS-1 with FADD and caspase-8. As shown in Figure 4b, both FADD and caspase-8 were associated with IPS-1. It is also shown that casapse-8 was strongly associated with IPS-1 than FADD. These results suggest that IPS-1 induces anoikis by the interaction with caspase-8 and FADD to activate caspase-8.
To evaluate whether IPS-1 expression is regulated during anoikis induction, we analyzed its expression before and after cell detachment. As shown in Figure 5a, IPS-1 expression was induced after detachment and Bim expression was also induced as shown earlier.26 As IPS-1 KO MEF cells are resistant to anoikis, it is suggested that upregulation of IPS-1 expression during detachment contributes to anoikis induction.
Detachment of the MDCK and MCF-10A cells led to the activation of caspase-8, which triggered mitochondrial dysfunction and activation of the effector caspase-3 and -7.24, 25 We found that caspase-8 was associated with IPS-1 and IPS-1 expression induced the cleavage of caspase-8 (Figures 1d, 4a and b). Therefore, we next examined whether the interaction of caspase-8 with IPS-1 is induced in the mitochondria after cell detachment. As shown in Figure 5b, procaspase-8 was associated with IPS-1 in a mitochondrial fraction after cell detachment.
In conclusion, IPS-1 was shown to be crucial for anoikis induction at the downstream of DAP3 by caspase-8 activation after cell detachment.
Materials and Methods
Cell culture and materials
MCF-10A cells were cultured as described earlier.26 HEK293, 293T cells, or MEFs from IPS-1-deficient mice were maintained in DMEM supplemented with 10% FCS, penicillin (50 U/ml), and streptomycin (50 μg/ml). IPS-1-deficient mice were generated by the standard gene targeting.21 The targeting vector was designed to disrupt two exons harboring the CARD-like domain of IPS-1, which is required for its function. Immunoprecipitation and immunoblotting were performed using anti-HA (Roche, Basel, Switzerland), anti-flag (Sigma, St Louis, MO, USA) antibody. Anti-Bim, anti-cleaved caspase-3, anti-cleaved caspase-8, anti-cleaved caspase-9 antibodies were purchased from Cell Signaling Technology, Danvers, MA, USA. Anti-FADD antibody (BD Transduction Laboratories, Franklin Lakes, NJ, USA), anti-IPS-1 (Cardif) antibody (Alexis Biochemicals, Rosen, Switzerland), anti-β-actin antibody (Chemicon, Temecula, CA, USA), and anti-mouse IPS-1 antibody (Kumar et al., 2006) were used for the western blotting analysis.
Transfections, immunoprecipitation, and immunoblotting
HEK293 or 293T cells (1 × 106) in 10 cm culture dishes were transiently transfected with 10 μg (total) of plasmid DNA with lipofectamine 2000. After 48 h, the cells were collected and suspended in lysis buffer containing 0.5% Nonidet P-40, 20 mM Tris-HCl buffer (pH 7.5), 1 mM EDTA, 150 mM NaCl, and protease inhibitor (Complete, Mini). Immunoprecipitations were performed using 10 μl of anti-FLAG antibody M2-conjugated-agarose (Sigma) or the other antibodies at 4 °C for 2 h after the cell lysates were precleared with 10 μl of protein A-agarose or protein G-agarose. The immune complexes were analyzed by SDS-PAGE and immunoblotted using the indicated antibodies after extensive washing by lysis buffer. MCF-10A cells were transfected with siRNA (20 nM) using oligofectamine (16 μl). At first, OPTI-MEM (500 μl) and oligofectamine were mixed and incubated at room temperature for 5 min. This solution was added to the solution including OPTI-MEM (500 μl) and each siRNA. Finally, this mixed solution was incubated at room temperature for 20 min and added to each well in which MCF-10A cells were cultured. These cells were cultured with growth medium containing 0.5% methylcellulose (Sigma) in poly-HEMA-coated culture plates for the indicated times. Cell lysates were analyzed by immunoblotting with antibodies for IPS-1, Bim, or β-actin. For the immunoprecipitation, cytosolic and mitochondrial fractions were prepared from MCF-10A cells (5 × 107) in the attached condition or in suspension culture for 6 h, using the mitochondrial/cytosol fractionation kit (Alexis Biochemicals). Mitochondrial lysates were immunoprecipitated with rabbit anti-human IPS-1 antibody (2 μg) and protein A/G-Sepharose beads overnight at 4 °C. Immunoprecipitated proteins were analyzed by western blotting using antibodies for caspase-8 or IPS-1.
DNA fragmentation assay
After cells (5 × 105) were suspended in 100 μl of 10 mM Tris-HCl (pH 7.5) buffer containing 10 mM EDTA, 0.5% Triton X-100, and RNase A (200 μg/ml), cell lysates were incubated for 10 min at the room temperature. These cell lysates were centrifuged at 1.5 × 104 r.p.m. for 20 min at 4 °C, the supernatant was incubated for 1 h at 37 °C. Then, 1 μl of proteinase K (20 mg/ml) was added, and the solution was incubated for additional 1 h. DNA was precipitated with 50% isopropanol containing 420 mM NaCl, dissolved in TE solution, separated in 2% agarose gels, and stained with ethidium bromide. The stained gels were photographed on a UV transilluminator.
Assay for the activity of caspase-3/7, caspase-8, or -9
Activity of caspase-3/7, caspase-8, or -9 was measured using caspase-Glo 3/7, caspase-Glo 8, or caspase-Glo 9 detection assay kit (Promega, Madison, WI, USA). These reagents (Promega) were equilibrated to the room temperature and mixed well. Each reagent (100 μl) was added to each well of 96-well plates, in which attached cells or detached cells were cultured. Each plate was gently mixed and incubated at room temperature for 30 min to 1 h. The luminescence of each sample was measured by a plate-reading luminometer. Wt and IPS-1 KO MEFs were seeded into 96-well plates at a density of 5 × 103 cells/well in triplicate wells. After 24 h, cells were treated with 10 μg/ml cycloheximide (CHX) alone or in combination with TNF-α (0.05 μg/ml), anti-Fas Antibody (Jo2, 200 ng/ml), or TRAIL (0.2 μg/ml). After these stimulations, caspase-3/7 activity was assessed by caspase-3/7 Glo reagents.
Anoikis assays
Anoikis assays were performed using established procedures.11 WT and IPS-1 KO MEFs were seeded into the wells of 96-well plates with or without poly-HEMA-coating at a density of 5 × 103 cells/well in triplicate wells. Cells after detachment were cultured in 1% FBS medium with methylcellulose (0.5%). Cells (2.5 × 104) were analyzed for apoptosis induction using the cell death detection ELISA kit (Roche). The analysis at each time point was performed in duplicate and each experiment was carried out at least three times. For the MTT assay, cells were cultured in 96 well-plates for 24 h. Then, 10 μl of MTT assay solution of Cell Counting Kit-8 (Wako Chem. Osaka, Osaka, Japan) was added to each well and after 3 h the absorbance of the solution was measured by spectrophotometer.
Plasmids and RNAi
Flag-tagged and myc-tagged expression vectors for human IPS-1 and mutants (IPS-1N, IPS-1C, and IPS-1ΔMito) were used as described earlier.16 Flag-tagged and HA-tagged expression vectors for human DAP3, FADD, and caspase-8 were used as described earlier.23 For siRNA experiments, double-stranded RNA duplexes composed of 21-nucleotide sense and antisense oligonucleotides were synthesized by iGENE and used as described earlier.16 Knockdown of IPS-1 expression was verified by western blotting analysis using anti-IPS-1 antibody.
Abbreviations
ECM:
extracellular matrixDAP3:
death-associated protein 3IPS-1:
interferon-β promoter stimulator 1MEF:
mouse embryonic fibroblastTNF-α:
tumor necrosis factor αTRADD:
TNF receptor 1-associated death domain proteinFADD:
FAS-associated death domainTRAIL:
TNF-related apoptosis inducing ligand.
References
- Jacobson MD, Weil M, Raff MC . Programmed cell death in animal development. Cell 1997; 88: 347–354.CAS PubMed Google Scholar
- Wajant H . The Fas signaling pathway: more than a paradigm. Science 2002; 296: 1635–1636.Article CAS Google Scholar
- Opferman JT, Korsmeyer SJ . Apoptosis in the development and maintenance of the immune system. Nat Immunol 2003; 4: 410–415.Article CAS Google Scholar
- Ricci JE, Gottlieb RA, Green DR . Caspase-mediated loss of mitochondrial function and generation of reactive oxygen species during apoptosis. J Cell Biol 2003; 160: 65–75.Article CAS Google Scholar
- von Ahsen O, Renken C, Perkins G, Kluck RM, Bossy-Wetzel E, Newmeyer DD . Preservation of mitochondrial structure and function after Bid- or Bax-mediated cytochrome c release. J Cell Biol 2000; 150: 1027–1036.Article CAS Google Scholar
- Chipuk JE, Fisher JC, Dillon CP, Kriwacki RW, Kuwana T, Green DR . Mechanism of apoptosis induction by inhibition of the anti-apoptotic BCL-2 proteins. Proc Natl Acad Sci 2008; 105: 20327–20332.Article CAS Google Scholar
- Luo X, Budihardjo I, Zou H, Slaughter C, Wang X . Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell 1998; 94: 481–490.Article CAS Google Scholar
- Li H, Zhu H, Xu CJ, Yuan J . Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell 1998; 94: 491–501.Article CAS Google Scholar
- Kim HR, Chae HJ, Thomas M, Miyazaki T, Monosov A, Monosov E et al. Mammalian dap3 is an essential gene required for mitochondrial homeostasis in vivo and contributing to the extrinsic pathway for apoptosis. FASEB J 2007; 21: 188–196.Article CAS Google Scholar
- Meredith JE, Fazeli B, Schwartz MA . The extracellular matrix as a cell survival factor. Mol Biol Cell 1993; 4: 953–961.Article CAS Google Scholar
- Frisch SM, Francis H . Disruption of epithelial cell-matrix interactions induces apoptosis. J Cell Biol 1994; 124: 619–626.Article CAS Google Scholar
- Frisch SM, Screaton RA . Anoikis mechanisms. Curr Opin Cell Biol 2001; 13: 555–562.Article CAS Google Scholar
- Grossmann J . Molecular mechanisms of ‘detachment-induced apoptosis—Anoikis’. Apoptosis 2002; 7: 247–260.Article CAS Google Scholar
- Miyazaki T, Shen M, Fujikura D, Tosa N, Kim HR, Kon S et al. Functional role of death-associated protein 3 (DAP3) in anoikis. J Biol Chem 2004; 279: 44667–44672.Article CAS Google Scholar
- Lad SP, Yang G, Scott DA, Chao TH, Correia Jda S et al. Identification of MAVS splicing variants that interfere with RIGI/MAVS pathway signaling. Mol Immunol 2008; 45: 2277–2287.Article CAS Google Scholar
- Kawai T, Takahashi K, Sato S, Coban C, Kumar H, Kato H et al. IPS-1, an adaptor triggering RIG-I- and Mda5-mediated type I interferon induction. Nat Immunol 2005; 6: 981–988.Article CAS Google Scholar
- Xu LG, Wang YY, Han KJ, Li LY, Zhai Z, Shu HB . VISA is an adapter protein required for virus-triggered IFN-beta signaling. Mol Cell 2005; 19: 727–740.Article CAS Google Scholar
- Meylan E, Curran J, Hofmann K, Moradpour D, Binder M, Bartenschlager R et al. Cardif is an adaptor protein in the RIG-I antiviral pathway and is targeted by hepatitis C virus. Nature 2005; 437: 1167–1172.Article CAS Google Scholar
- Seth RB, Sun L, Ea CK, Chen ZJ . Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF- κB and IRF 3. Cell 2005; 122: 669–682.Article CAS Google Scholar
- Takahashi K, Kawai T, Kumar H, Sato S, Yonehara S, Akira S . Roles of caspase-8 and caspase-10 in innate immune responses to double-stranded RNA. J Immunol 2006; 176: 4520–4524.Article CAS Google Scholar
- Kumar H, Kawai T, Kato H, Sato S, Takahashi K, Coban C et al. Essential role of IPS-1 in innate immune responses against RNA viruses. J Exp Med 2006; 203: 1795–1803.Article CAS Google Scholar
- Kissil J, Cohen O, Raveh T, Kimchi A . Structure-function analysis of an evolutionary conserved protein, DAP3, which mediates TNF-α and Fas-induced cell death. EMBO J 1999; 18: 353–362.Article CAS Google Scholar
- Miyazaki T, Reed JC . A GTP binding adapter protein couples TRAIL receptors to apoptosis-inducing proteins. Nat Immunol 2001; 2: 493–500.Article CAS Google Scholar
- Frisch SM . Evidence for a function of death-receptor-related, death-domain-containing proteins in anoikis. Curr Biol 1999; 9: 1047–1049.Article CAS Google Scholar
- Rytömaa M, Martins LM, Downward J . Involvement of FADD and caspase-8 signalling in detachment-induced apoptosis. Curr Biol 1999; 9: 1043–1046.Article Google Scholar
- Reginato MJ, Mills KR, Paulus JK, Lynch DK, Sgroi DC, Debnath J et al. Integrins and EGFR coordinately regulate the pro-apoptotic protein Bim to prevent anoikis. Nat Cell Biol 2003; 5: 733–740.Article CAS Google Scholar
Acknowledgements
This work was supported by Grants-in-Aid for Scientific Research from Ministry of Education, Culture, Sports, Science & Technology, Grants from Japan Health Sciences Foundation, and Grant of the Program of Founding Research Centers for Emerging and Reemerging Infectious Diseases, MEXT Japan and Grants by the National Institutes of Health (GM61694 and AI5624).
Author information
Authors and Affiliations
- Department of Bioresources, Hokkaido University Research Center for Zoonosis Control,H-M Li, D Fujikura, T Harada, J Uehara, A Iwai & T Miyazaki
- Sapporo, Hokkaido, JapanH-M Li, D Fujikura, T Harada, J Uehara, A Iwai & T Miyazaki
- Laboratory of Host Defense, WPI Immunology Frontier Research Center, Osaka University, 3-1 Yamada-oka,T Kawai & S Akira
- Suita, Osaka, JapanT Kawai & S Akira
- Department of Host Defense, Research Institute for Microbial Diseases, Osaka University, 3-1 Yamada-oka,T Kawai & S Akira
- Suita, Osaka, JapanT Kawai & S Akira
- Burnham Institute for Medical Research, 10901 N. Torrey Pines Rd, La Jolla, CA, USAJ C Reed
Corresponding author
Additional information
Edited by HU Simon
Supplementary Information accompanies the paper on Cell Death and Differentiation website (http://www.nature.com/cdd)
Supplementary information
Supplementary Figures 1–7 (PDF 171 kb)
Supplementary Figures Legends (DOC 36 kb)
References
