Abstract
Candida albicans (C. albicans) cell wall beta-glucan has been considered as a potential agent in the treatment of cancers due to its anti-tumor properties. Therefore, in the present study, we investigated the anti-cancer effects of Candida cell wall beta-glucan on Lewis lung carcinoma cell line (LL/2) cells. Beta-glucan of C. albicans cell wall was extracted. LL/2 cell line was cultured, then sphere cells and parental cells were exposed to the different concentrations of beta-glucan extracted from C. albicans (10–6000 μg/ml), for 24, 48 and 72 h. Cytotoxicity of beta-glucan was assayed by MTT test, then RNA extracted from cells population (treated and untreated cells), cDNA synthetized and expression level of Sox2, Oct4, C-myc, Nanog genes were also investigated using Real-time methods. At optimal concentrations of 800 and 1000 μg/ml, the extracted beta-glucan showed a significant cytotoxic effect on both parental and sphere cell populations (p < 0.05). Real-time PCR analysis revealed a decreased expression of Oct4 and Sox2 genes in treatment of cells with beta-glucan compared with control group. Since the extracted beta-glucan showed an inhibitory effect on the expression of Oct4 and Sox2 genes involved in LL/2 metastasis, therefore, beta-glucan can be considered as an anti-tumor agent because of its anti-metastatic properties, however, more in vitro and in vivo studies are needed to provide further evidence on this topic in the future.
Similar content being viewed by others
Lung cancer and β-glucans: review of potential therapeutic applications
Article 16 March 2017
Long noncoding RNA MGC27382 inhibits proliferation and metastasis of non-small cell lung cancer cells via down-regulating AKT/GSK3β pathway
Article 05 July 2021
Anti-tumor effect of β-glucan from Lentinus edodes and the underlying mechanism
Article Open access29 June 2016
Explore related subjects
Discover the latest articles, books and news in related subjects, suggested using machine learning.
Introduction
Candida albicans (C. albicans) is a normal flora of skin and mucous membrane of human which could become pathogen in immunocompromised patients and causes a wide range of diseases [1, 2]. C. albicans cell wall consists of 90% carbohydrate (60% of beta-1,3 and beta-1, 6 glucan and 40% of mannan) and 10% protein [3, 4]. Beta-glucans are polysaccharides of D-glucose monomers linked by beta-1, 3 and beta-1, 4 bonds, which stimulate macrophages and dendritic cells by activating signaling pathways, subsequently the stimulated immune cells release inflammatory mediators such as IL-1, TNF and prostaglandin E2, and eventually the growth of cancer cells is inhibited and an appropriate condition is provided to kill the tumor cells [5,6,7,8]. Lung cancer is the most common cause of cancer deaths around the world due to its low 5-year survival rates (Less than 20%) [9]. The cause of the high mortality in people with lung cancer is the lack of timely diagnosis of disease, resistance to conventional treatments (chemotherapy, radiotherapy, etc.), metastasis, and recurrence of the disease. Studies have shown that these characteristics are due to the presence of cancer cell stem cells (CSCs) in tumor tissue of the lung with differentiation and self-renewal abilities [9,10,11]. Several studies have shown CSCs interfere with the metastasis process, which have become the biggest barrier to cancer treatment and the major cause of death in cancer patients [12, 13]. Consequently, conventional treatments of cancer, such as chemotherapy and radiotherapy, have many complications such as regional or systemic toxicity and drug resistance [14, 15]. To achieve a successful therapy, the conventional treatments should be combined with targeted new strategies against CSCs to prevent tumor recurrence and also provide an effective therapy with high-quality and low toxicity [16,17,18].Recently, several studies have long been considered the anti-cancer activity of beta-glucan as a natural component on different cancer cell line with immune-stimulatory and immunomodulatory potential therapeutic effect [19,20,21,22,23,24,25], and its ability to treat infectious diseases as well [26,27,28]. It is noteworthy that evidence toward anti-cancer effect of beta-glucan against LL/2 cell line and drug-resistance cells (Spheroid cells) is limited [14, 29]. Therefore, for filling this gap, the present study aimed to investigate the assessment of anti-cancer activity of Candida cell wall beta-glucan on resistant LL/2cancer cells in vitro.
Materials and methods
Culture condition and harvest of C. albicans
Standard strain of Candida albicans (C. albicans, ATTC10231) was used for beta- glucan extraction. For precise identification of Candida, PCR amplification of fungal rRNA genes was performed by using the universal ITS primers (ITS1–ITS4). C. albicans was cultured on Sabouraud dextrose Agar (SDA, Merck) at 35–37 °C for 72 h, and after reaching a maximum growth, the yeast was transferred to YPG broth medium contains 1% Yeast (Sigma Aldrich, USA), 2% peptone (Sigma Aldrich, USA), 2% glucose and incubated for 48 h at 37 °C in shaker condition(100 rpm).
Extraction of beta-glucan from C. albicans cell wall and its characterization
Beta- glucan was extracted from yeast cells according to a minor modification of the methods described [30, 31]. The yeast cells were harvested from YPD medium by centrifuging at 5000 rpm for 10 min. The sediment was collected and then lyophilized for 48 h (FDU-8606, Operon, Gimpo-si, Korea). Approximately 50 ml of distilled water, 0.04 g NaoH and 25 ml of sodium hypochlorite were added to 2.0 g of the obtained yeast powder, and then placed at 4 °C for 24 h. After this period, the mixture was centrifuged and 2 ml of ethanol–acetone added and then centrifuged under the same condition. To remove proteins, 1 ml of acetic acid and 1 ml of Dimethyl sulfoxide (DMSO, Sigma Aldrich, USA)) were added to 0.1 g of the precipitate and incubated in a hot water bath for 60 min.
Subsequently, the solution was centrifuged and equal proportions of ethanol-acetone were added to the supernatant liquid and placed overnight at 4 °C and centrifuged. Zymolyase (Sigma Aldrich, USA) dissolved in Phosphate-buffered saline (PBS 1×, PH 7.4) was added to the precipitate in order to break the polysaccharide bounds, and finally the enzyme activity was terminated by placing in a hot water bath for 5 min.
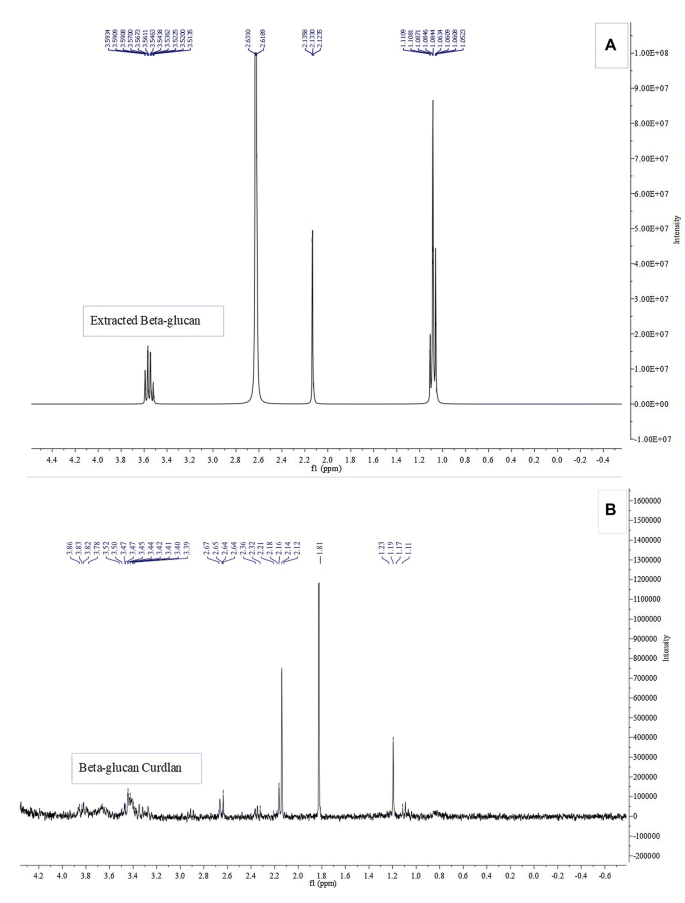
Proton magnetic resonance (H NMR) was used to examine the extracted beta-glucan. The sample was dissolved in deuterium oxide (D2O) and NMR spectrum was recorded on a Bruker AVANCE 300 FT-NMR spectrometer, resonating at 300 MHz (1H) and compared with the 1H NMR spectrum of beta- glucan standard form [32]
Cell culture and growth condition of LL/2 cell line
LL/2 cell line (murine LL/2 Lewis lung carcinoma cell line) obtained from the National Cell Bank of Iran, Pasteur Institute of Iran was cultured under sterile conditions in Dulbecco’s modified Eagle’s medium (DMEM; Gibco, Invitrogen, USA), 10% fetal bovine serum (FBS; Gibco, Invitrogen, USA), 2 mM l-glutamine, 100 μg/ml streptomycin and 100 U/ml penicillin and incubated at 37 °C with 5% CO2.
Cell viability assay
Approximately 2 × 105 cells were added to a tissue culture flask (SPl Life Bioscience, Korea) containing 5 ml of the complete medium and incubated at 37°. After reaching confluency (80–90%), the cells were passaged and counted as following. 5 μl of cell suspension was mixed with 5 μl of trypan blue color. Then cells were counted using Hemacytometric slide. Cells that did not take up the trypan blue color were considered as living cells [33]. Then the percentage of cell survival was calculated by the following formula:
Colony-formation assay
For this purpose approximately 80 single and live cells of each population (parental cells and spheroid cells) was seeded on six-well culture plates containing complete medium. After 10 days, the cell colonies were fixed with 4% paraformaldehyde and stained with 0.05% crystal violet (Sigma Aldrich, USA). The number of colonies, more than fifty cells in each well, was counted and the colony-forming potential calculated by the method described by Franken et al. [34].
Enrichment and passage of spheroid cells
The number of 85 × 104 cells was plated in each well of a 24-well ultra-low attachment plate (SPl Life Bioscience, Korea) containing 800 μl of serum-free medium. The plate was incubated for 24 h at 37 °C, and gently pipetted several times during day. After 3 to 5 days, spheres (cells) were enriched and harvested. Cell populations was washed by adding 1 ml of PBS and incubated for 2 min at 37 °C. After centrifugation at 1500 rpm for 5 min, 1 ml of trypsin and also 2 ml of serum-free DMEM medium were added to cell sediment and pipetting was done slowly. After centrifugation and adding the serum-free DMEM medium to cell sediment, cell suspension was added to each well of 24-well microplate and incubated at 37 °C.
Cytotoxicity effect of beta-glucan on the parental cells and sphere cells
Due to insolubility of the extracted beta-glucan in sterilized distilled water and PBS, 0.1% DMSO concentration [35], was used for solving the extracted β-glucan. 0.005 g beta-glucan was dissolved in 0.1% DMSO to obtain the final concentration of 5000 μg/ml. After that, serial dilutions of beta-glucan were prepared at concentrations of 100, 200, 400, 800 and 1000 μg/l. For cytotoxicity assay, a total of 104 parental tumor cells were added to each well of 24-well microplate (SPl Life Bioscience, Korea) containing 100 μl of complete DMEM medium and incubated for 24 h at 37 °C. Then, the wells were evacuated and 75 μl of complete culture medium and 25 μl of various concentrations of the extracted beta-glucan were added to each well and incubated at 37° for 48 h. After the 48-h time period, the wells were evacuated, then 90 μl of serum-free medium and 10 μl of MTT(3-[4,5-Dimethyl-2 thiazoyl][Geller, 2019 #49]-2,5-diphenyl-2Htetrazolium bromide, Sigma Aldrich,USA) dissolved in PBS at 0.5 mg/ml final concentration were added to each well and microplate was incubated at 37° for 4 h. Then, 100 μl of DMSO was added to each well, and finally, the microplate was read by the ELISA reader at a wavelength of 570 nm.
In addition, about 85 × 104 spheroid cells were seeded in to each well of 24-well ultra-low attachment plate containing 800 μl of serum-free medium. After 24-h incubation at 37° and pipetting, treatment of sphere cells with different concentrations of the extracted beta-glucan and cytotoxicity assay were performed with the same method. Each experiment was carried out three times.
Comparison of absorbance values untreated cells (control group) with treated cells showed a reduction in the rate of cell proliferation and finally the percentage of cell survival was calculated according to the following formula:
where A = absorbance.
Total RNA extraction
Extraction of total RNA from the parental and sphere cell populations (treated cells and the control group (was carried out by the Trizol method according to the manufacturer’s protocol (Sigma Aldrich, USA). In order to elimination of DNA, RNase-free DNase I (Fermentas, Germany) was added to the obtained total RNA and then the presence and purity of the samples were determined by using spectrophotometry and agarose gel electrophoresis.
cDNA synthesis and Real-time PCR
cDNA was synthetized using RevertAidTM first Strand cDNA Synthesis Kit (Fermentas, Germany) according to the manufacturer’s protocol. Briefly, 1 μl of random hexamer primers, 2 μl of RNA templates and 12 μl of RNase-free water were added and incubated for 5 min at 65 °C. Then 4 μl of buffer (5×), 2 μl of dNTP solution (10 mM), 1 μl of RNase inhibitor and 1 μl of RTase (only to the tube containing the main RNA sample) were added. The samples were well mixed and incubated for 5, 60 and 5 min at 25, 42 and 70 °C, respectively. For quantitative analysis of gene expression level of Sox2, Oct4, C-myc and Nanog, 6 μl distilled water, 1 μl of each the forward and reverse primers (5 pmol/μl), 10 μl of SYBR ®Premix Ex Taq™II (RR081Q, Takara Bio., Inc.), and 2 μl of the diluted cDNA sample were mixed, then the prepared sample was placed in to in a Rotor-Gene™ 6000 Real-time PCR System (Corbett Life Science, Sydney, Australia). Average expression of GAPDH gene was used for normalizing expression. The sequence-specific primers (PCR-SSP) used in the study are shown in Table 1.Table 1 Sequence-specific primers used in the study
Statistical analysis
All results are expressed as the mean ± SEM of at least three independent experiments. Statistical analysis was done using SPSS statistical software (version 21.0) (SPSS Inc., Chicago, IL, USA). Statistical comparisons between the control and treatment groups were assessed by Student’s t-test and one-way ANOVA was also used for multiple comparisons. All P-values of < 0.05 were considered statistically significant.
Results
Beta-glucan characterization using HNMR method
Beta-glucan was extracted from the Candida yeast cells and HNMR method was used to validate the presence of beta-glucan. To assess β-(1 → 3)-d-glucan chemical structure, beta-glucan was dissolved in deuterium water (deuterium oxide) and its structure evaluated by NMR assay and then compared and confirmed with the spectral data of the beta-glucan purchased from sigma Aldrich Company, USA (Fig. 1).
Optimization of the spheroid cells number
Spheroid-formation assay was performed by using the 24-wells ultra-low attachment plate. To determine the optimal number of cells/well, serial dilutions of cell suspension in FBS-free medium (150,000, 250,000,750,000, 850,000, 900,000 and 1,000,000) were prepared and after a 24-h incubation, the wells were checked by inverse optical microscope. Finally, the number of 850,000 cells was determined as the most efficient number of cells in each well. After incubation at 37 °C for 5 to 7 days, cells were transferred to 96-well plate. In the first and third cell passages, the cytotoxicity effect of the extracted beta-glucan on the spheroid cells was evaluated using the MTT assay.
MTT assay optimization for parental and spheroid cells viability determination
Beta-glucan effects on LL/2 cells were evaluated in the presence of three factors (cell number, beta-glucan concentration and incubation time) and then the optimal state was determined for each of the items. At first, 1500, 2500, 5000, 6000 and 10,000 cells/wells were seeded in to a 96-wells plate.
In the next step, different concentrations of beta-glucan including 10, 25, 50, 75, 100, 150, 200, 400, 600, 800, 1000, 1500, 2000, 2500, 3000, 3500, 4000 μg/ml and also 4500, 5500, 6000 μg/ ml were added to the wells containing parental cells and spheroid cells. In order to obtain the optimal time for cell incubation, various time intervals (24, 48, 72 and 120 h) were assayed. According to our findings in the MTT assay (to determine cytotoxicity following exposure to the extracted β-glucan), number of 10,000 cells/well, the 100–1000 μg/ml beta-glucan concentrations and the 48-h incubation were selected for treatment-resistant spheroid cell population.
Cytotoxic effect of extracted beta-glucan on the parental cells and spheroid cells
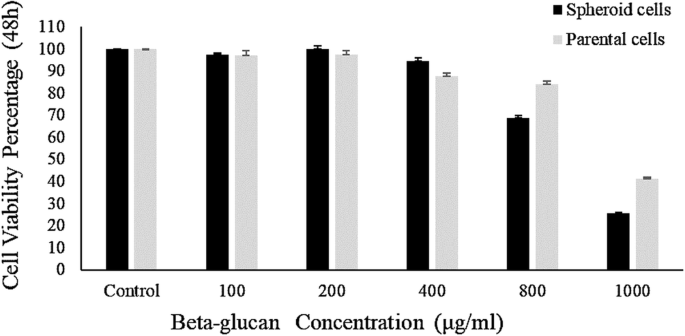
To compare the effect of the extracted beta-glucan and standard beta-glucan on parental and spheroid cell populations, cytotoxicity test was carried out on both cell lines at different concentrations of the extracted beta-glucan and standard beta-glucan (100, 200, 400, 800, 1000 μg/ml).
The findings expressed no significant difference in the cytotoxicity effect of the extracted beta-glucan and standard beta-glucan (p > 0.05). Cytotoxicity findings showed that at concentrations of 100, 200 and 400 μg/ml of extracted beta-glucan, there was no significant difference in cytotoxicity on both the parental cells and spheroid cells, however, at 800 and 1000 μg/ml beta-glucan concentrations, it was considerably significant (p < 0.05). In addition, the cytotoxic effect of the beta-glucan on the spheroid cells was significantly greater than that on the parental cells compared with the control group (untreated cells) (p-value of 800 μg/ml = 0.017, p-value of 1000 μg/ml = 0.0009). Also, the extracted beta-glucan at 1000 μg/ml concentration had a significant cytotoxic effect on parental cells (p = 0.005) and spheroid (p = 0.0001) compared with control group. The results are shown in Fig. 2.
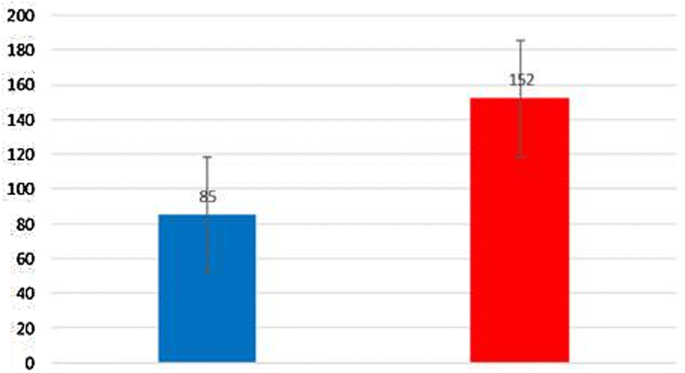
Colony-forming assay of parental cells and spheroid cells
At 800 and 1000 μg/ml concentrations of the extracted beta-glucan, a decrease in tumorgenicity was observed in both parental and spheroid cell populations. However, this effect on spheroid cells was higher than on parental cells (Fig. 3).
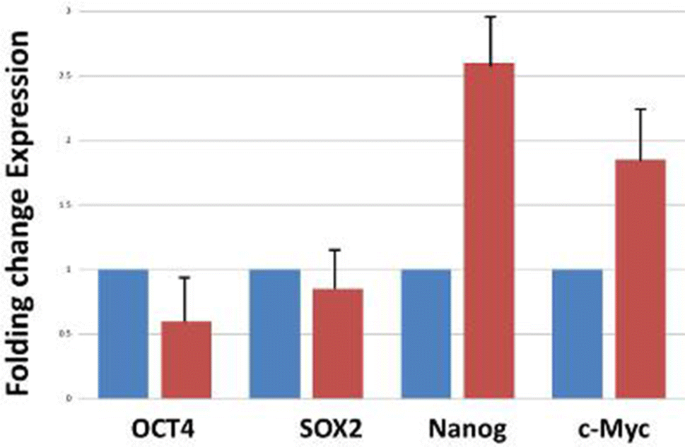
Gene expression level of GAPDH, Oct4, Sox2, Nanog, and C-myc using Real-time PCR
At first, total RNA was extracted from cell populations (treated and untreated cells) and then cDNA was Synthetized (Fig. 4). Gene expression level of Oct4, Sox2, Nanog, and C-myc was assessed using Real-Time PCR. The results showed decreased expressions of Oct4 and Sox2 genes and increased expressions of Nanog and c-Myc genes in treated spheroid cells compared with the control group (Fig. 4).
Discussion
Although previous studies have been conducted to investigate the anti-tumor effect of the beta-glucan extracted from fungi on various types of cancers [36], the effects of Candida beta-glucan on lung carcinoma cells have not been evaluated so far. Our findings indicated that the function of the beta-glucan extracted from C. albicans using the modified method has been consistent with standard methods, these findings surprisingly demonstrated the cytotoxic effects of the extracted beta-glucan on LL/2 cell line in vitro. According to the obtained results, the extracted beta-glucan at concentrations lower than 100 μg/ml (10, 25, 50, 75) did not show any cytotoxicity against LL/2 cells (p < 0.05), whereas at concentrations higher than 100 μg/ml (1000 and 800 μg/ml), this effect was significantly increased (p < 0.05). Interestingly, treatment of cells with 800 and 1000 μg/ml concentrations of β-glucan under conditions of 48-h incubation and 10,000 cells/well, were considered in our study.
In fact, we found that beta-glucan at 800 and 1000 μg/ml concentrations had a significant cytotoxic effect on LL/2 cells compared with the control groups (p value of parental = 0.005 and p value of Sphere = 0.0001). The survival percentages of parental cells exposed to 800 and 1000 μg/ml concentrations of beta glugcan were 88% and 40%, respectively, and those of spheroid cells at the same concentrations were 60% and 30%, respectively. This difference in the response of spheroid and parental cells to beta-glucan may be result from the heterogeneity of the tumor cell population [37], which leads to different response to the treatment. In addition, this effect at 1000 μg/ml concentration of beta-glucan (in both parental and spheroid cell populations) was greater than that at 800 μg/ml concentration, which confirms the dose-dependent effect of beta-glucan on LL/2 cell line.
To investigate the stem cell self-renewal, colony formation by spheroid cell population in comparison to parental cell population was measured. Spheroids have the characteristics of stem cells such as continuous self-renewal, drug resistance, high proliferative potential, drug resistance, high tumorigenesis capacity and overexpression of Oct4, Sox2, Nanog genes [38, 39]. Because of the contribution of this cell population to tumor generation, progression and chemotherapy resistance, they have received much attention [40, 41]. Along with findings, extracted beta-glucan caused significant decrease in tumorigenic ability of the spheroid cell population, while this effect on the parental cell population was less.
To evaluate the changes in gene expression of the parental and spheroid cell populations after exposure to cytotoxic concentrations of beta-glucan (800 and 1000 μg/ml), we determined the effects of beta-glucan on the expression patterns of Oct4, Sox2, Nanog, c-myc genes in a quantitative measurement alongside the House-keeping gene (GAPDH). Real time-PCR indicated a significant decrease in expression of both Oct4 and Sox2 genes after exposure of cells to beta-glucan compared with the group control.
It is surprising that beta-glucan can be considered as a potential promising agent in metastasis inhibition of cancer cells, since it caused decreased expression of two genes that play a crucial role in metastasis and tumorgenesis of treated cells [42,43,44,45]. In fact, extracted beta-glucan, in addition to cytotoxicity properties against LL/2 cell line, at the level of gene expression in cancer cells has also been able to exhibit its anti-cancer effects. Our findings are agreement with similar previous studies Consistently, Yoon, et al. showed that the beta-glucan extracted from Saccharomyces cerevisiae has inhibitory effects on B16-BL6 melanoma cells, and pulmonary metastasis model of colon carcinoma 26-M3.1 [46]. In a similar study by Queiroz et al., exposure of MCF-7 cells to beta-glucan of Botryosphaeria rhodina led to a decrease in the percentage of viable cells, an increased expression of p53 gene was also observed [47]. The highest cytotoxicity effects of beta-glucan in their study were observed at concentrations of 1500 μg/ml, which is comparable with our study because beta-glucan could significantly exert its cytotoxic effects on cells (p < 0.05) at 800 and 1000 μg/ml concentrations.
Noticeably, in spite of our expectation, beta- glucan at concentrations higher than 1000 μg/ml did not show any cytotoxic effects on cancer cells, it can be assumed that in higher concentrations, the beta-glucan may exert adverse effects without efficacy.
In fact, the cytotoxic effect of beta-glucan on LL/2 cell line in our study was in a dose-dependent manner because these effects were not apparent at higher concentrations, which is comparable with the study by Queiroz et al. [47]. In agreement with our findings, Kobayashi et al. specified the significant inhibitory effect of beta-glucan on the invasion of tumoral cell lines and, decreased expression of uPA gene as inhibitor gene in tumorgensis of cell lines as well [48]. Finally extracted beta-glucan exerted the higher cytotoxic effects on spheroid cells in comparison to parental cells, as a result, it can be suggested that beta- glucan of Candida cell wall is an effective combination agent against treatment-resistant tumor cells.
Conclusion
Taken together, it can be concluded that beta-glucan might be appraised as an effective dose-dependent anti-cancer agent, because the beta-glucan extracted from the Candida albicans cell wall is capable of inhibiting the growth of LL/2 cell line with a dose-dependent cytotoxic effects. Also, to confirm the cytotoxic effects of beta-glucan, it is necessary to carry out extensive studies for investigating the effects of beta -glucan on a different type of cancer invitro and invivo in the future.
References
- Romo JA, Kumamoto CA (2020) On commensalism of Candida. J Fungi 6(1):16Google Scholar
- Kadosh D (2019) Regulatory mechanisms controlling morphology and pathogenesis in Candida albicans. Curr Opin Microbiol 52:27–34PubMed Google Scholar
- Fesel PH, Zuccaro A (2016) beta-glucan: crucial component of the fungal cell wall and elusive MAMP in plants. Fungal Genet Biol 90:53–60. https://doi.org/10.1016/j.fgb.2015.12.004CAS PubMed Google Scholar
- Gow NA, Hube B (2012) Importance of the Candida albicans cell wall during commensalism and infection. Curr Opin Microbiol 15(4):406–412. https://doi.org/10.1016/j.mib.2012.04.005CAS PubMed Google Scholar
- Vetvicka V, Vetvickova J (2018) Glucans and cancer: comparison of commercially available beta-glucans—Part IV. Anticancer Res 38(3):1327–1333. https://doi.org/10.21873/anticanres.12355CAS PubMed Google Scholar
- Yin M, Zhang Y, Li H (2019) Advances in research on immunoregulation of macrophages by plant polysaccharides. Front Immunol 10:145CAS PubMed PubMed Central Google Scholar
- Kim HS, Park KH, Lee HK, Kim JS, Kim YG, Lee JH, Kim KH, Yun J, Hwang BY, Hong JT (2016) Curdlan activates dendritic cells through dectin-1 and toll-like receptor 4 signaling. Int Immunopharmacol 39:71–78CAS PubMed Google Scholar
- Thwe PM, Fritz DI, Snyder JP, Smith PR, Curtis KD, O’Donnell A, Galasso NA, Sepaniac LA, Adamik BJ, Hoyt LR (2019) Syk-dependent glycolytic reprogramming in dendritic cells regulates IL-1β production to β-glucan ligands in a TLR-independent manner. J Leukoc Biol 106(6):1325–1335CAS PubMed PubMed Central Google Scholar
- Suresh R, Ali S, Ahmad A, Philip PA, Sarkar FH (2016) The role of cancer stem cells in recurrent and drug-resistant lung cancer. In: Ahmad A, Gadgeel SM (eds) Lung cancer and personalized medicine: novel therapies and clinical management. Springer, Cham, pp 57–74Google Scholar
- Heng WS, Gosens R, Kruyt FA (2019) Lung cancer stem cells: origin, features, maintenance mechanisms and therapeutic targeting. Biochem Pharmacol 160:121–133CAS PubMed Google Scholar
- Raniszewska A, Polubiec-Kownacka M, Rutkowska E, Domagala-Kulawik J (2019) PD-L1 expression on lung cancer stem cells in metastatic lymph nodes aspirates. Stem Cell Rev Rep 15(2):324–330CAS PubMed Google Scholar
- Geiger TR, Peeper DS (2009) Metastasis mechanisms. BBA 1796(2):293–308CAS PubMed Google Scholar
- Das PK, Pillai S, Rakib MA, Khanam JA, Gopalan V, Lam AK, Islam F (2020) Plasticity of cancer stem cell: origin and role in disease progression and therapy resistance. Stem Cell Rev Rep 16:397–412PubMed Google Scholar
- Roudi R, Mohammadi S, Roudbary M, Mohsenzadegan M (2017) Lung cancer and β-glucans: review of potential therapeutic applications. Invest New Drugs. https://doi.org/10.1007/s10637-017-0449-9PubMed Google Scholar
- Nassar D, Blanpain C (2016) Cancer stem cells: basic concepts and therapeutic implications. Annu Rev Pathol 11:47–76CAS PubMed Google Scholar
- Al-Hajj M, Becker MW, Wicha M, Weissman I, Clarke MF (2004) Therapeutic implications of cancer stem cells. Curr Opin Genet Dev 14(1):43–47CAS PubMed Google Scholar
- Soltanian S, Matin MM (2011) Cancer stem cells and cancer therapy. Tumor Biol 32(3):425–440Google Scholar
- Guha D, Banerjee S, Mukherjee S, Dutta A, Das T (2020) Reactive oxygen species: friends or foes of lung cancer? In: Chakraborti S, Parinandi N, Ghosh R, Ganguly N, Chakraborti T (eds) Oxidative stress in lung diseases. Springer, Singapore, pp 331–352Google Scholar
- Del Cornò M, Gessani S, Conti L (2020) Shaping the innate immune response by dietary glucans: any role in the control of cancer? Cancers 12(1):155PubMed Central Google Scholar
- Geller A, Shrestha R, Yan J (2019) Yeast-derived β-glucan in cancer: novel uses of a traditional therapeutic. Int J Mol Sci 20(15):3618CAS PubMed Central Google Scholar
- Bose N, Gorden K, LEONARDO S, GRAFF J, Qiu X, KANGAS T, FRASER KA, JONAS AB, OTTOSON N, Fulton R (2019) Beta-glucan in combination with anti-cancer agents affecting the tumor microenvironment. Google Patents
- Sima P, Richter J, Vetvicka V (2019) Glucans as new anticancer agents. Anticancer Res 39(7):3373–3378PubMed Google Scholar
- Venturella G, Saporita P, Gargano ML (2019) The potential role of medicinal mushrooms in the prevention and treatment of gynecological cancers: a review. Int J Med Mushrooms 21(3):225–235PubMed Google Scholar
- Baldassano S, Accardi G, Vasto S (2017) Beta-glucans and cancer: the influence of inflammation and gut peptide. Eur J Med Chem 142:486–492CAS PubMed Google Scholar
- Zhang M, Kim JA, Huang AY-C (2018) Optimizing tumor microenvironment for cancer immunotherapy: β-glucan-based nanoparticles. Front Immunol 9:341PubMed PubMed Central Google Scholar
- Medina-Gali RM, del Mar O-V, Mercado L, Novoa B, Coll J, Perez L (2018) Beta-glucan enhances the response to SVCV infection in zebrafish. Dev Comp Immunol 84:307–314CAS Google Scholar
- Sun X, Gao Y, Ding Z, Zhao Y, Yang Y, Sun Q, Yang X, Ge W, Xu X, Cheng R (2020) Soluble beta-glucan salecan improves vaginal infection of Candida albicans in mice. Int J Biol Macromol 148:1053–1060CAS PubMed Google Scholar
- Nassar SA, Mohamed AM, Sedky D, El-Shemy A, Allam AM (2018) Oral and intraperitoneal administration of β-glucan and its immunomodulatory effect against staphylococcus aureus infection in rats. Int J Pharm Phytopharmacol Res 8(2):1–7CAS Google Scholar
- Saravanakumar K, Jeevithan E, Hu X, Chelliah R, Oh D-H, Wang M-H (2020) Enhanced anti-lung carcinoma and anti-biofilm activity of fungal molecules mediated biogenic zinc oxide nanoparticles conjugated with β-D-glucan from barley. J Photochem Photobiol, B 203:111728CAS Google Scholar
- Lee JN, Lee DY, Ji IH, Kim GE, Kim HN, Sohn J, Kim S, Kim CW (2001) Purification of soluble beta-glucan with immune-enhancing activity from the cell wall of yeast. Biosci Biotechnol Biochem 65(4):837–841. https://doi.org/10.1271/bbb.65.837CAS PubMed Google Scholar
- Nasrollahi Z, Roudbar Mohammadi S, Atyabi F, Zuhair Sarraf H, Yadegari MH, Esfandyari M, Mollarazi E (2013) A rapid method for extraction of water soluble β(1,3) glucan from the cell wall of Candida albicans. Pathobiol Res 16(1):89–97Google Scholar
- Kim YT, Kim EH, Cheong C, Williams DL, Kim CW, Lim ST (2000) Structural characterization of beta-D-(1 –%3e 3, 1 –%3e 6)-linked glucans using NMR spectroscopy. Carbohydr Res 328(3):331–341. https://doi.org/10.1016/s0008-6215(00)00105-1CAS PubMed Google Scholar
- Patel S, Gheewala N, Suthar A, Shah A, Patel S (2008) In-Vitro cytotoxicity activity of Solanum Nigrum extract against Hela cell line and Vero cell line. Int J Pharm Pharm Sci 1:38–46Google Scholar
- Franken NA, Rodermond HM, Stap J, Haveman J, van Bree C (2006) Clonogenic assay of cells in vitro. Nat Protoc 1(5):2315–2319. https://doi.org/10.1038/nprot.2006.339CAS PubMed Google Scholar
- Qi W, Ding D, Salvi RJ (2008) Cytotoxic effects of dimethyl sulphoxide (DMSO) on cochlear organotypic cultures. Hear Res 236(1–2):52–60CAS PubMed Google Scholar
- Albeituni SH, Ding C, Liu M, Hu X, Luo F, Kloecker G, Bousamra M 2nd, Zhang HG, Yan J (2016) Yeast-derived particulate beta-glucan treatment subverts the suppression of myeloid-derived suppressor cells (MDSC) by inducing polymorphonuclear MDSC apoptosis and monocytic MDSC differentiation to APC in cancer. J Immunol 196(5):2167–2180. https://doi.org/10.4049/jimmunol.1501853CAS PubMed PubMed Central Google Scholar
- Shackleton M, Quintana E, Fearon ER, Morrison SJ (2009) Heterogeneity in cancer: cancer stem cells versus clonal evolution. Cell 138(5):822–829. https://doi.org/10.1016/j.cell.2009.08.017CAS PubMed Google Scholar
- Abugomaa A, Elbadawy M, Yamawaki H, Usui T, Sasaki K (2020) Emerging roles of cancer stem cells in bladder cancer progression, tumorigenesis, and resistance to chemotherapy: a potential therapeutic target for bladder cancer. Cells 9(1):235PubMed Central Google Scholar
- Takahashi K, Asano N, Imatani A, Kondo Y, Saito M, Takeuchi A, Jin X, Saito M, Hatta W, Asanuma K (2020) Sox2 induces tumorigenesis and angiogenesis of early stage esophagealsquamous cell carcinoma through secretion of Suprabasin. Carcinogenesis. https://doi.org/10.1093/carcin/bgaa014PubMed Google Scholar
- Liao J, Qian F, Tchabo N, Mhawech-Fauceglia P, Beck A, Qian Z, Wang X, Huss WJ, Lele SB, Morrison CD, Odunsi K (2014) Ovarian cancer spheroid cells with stem cell-like properties contribute to tumor generation, metastasis and chemotherapy resistance through hypoxia-resistant metabolism. PLoS ONE 9(1):e84941. https://doi.org/10.1371/journal.pone.0084941CAS PubMed PubMed Central Google Scholar
- Liu J, Ma L, Xu J, Liu C, Zhang J, Liu J, Chen R, Zhou Y (2013) Spheroid body-forming cells in the human gastric cancer cell line MKN-45 possess cancer stem cell properties. Int J Oncol 42(2):453–459. https://doi.org/10.3892/ijo.2012.1720CAS PubMed Google Scholar
- Darini CY, Pisani DF, Hofman P, Pedeutour F, Sudaka I, Chomienne C, Dani C, Ladoux A (2012) Self-renewal gene tracking to identify tumour-initiating cells associated with metastatic potential. Oncogene 31(19):2438–2449. https://doi.org/10.1038/onc.2011.421CAS PubMed Google Scholar
- Xiang R, Liao D, Cheng T, Zhou H, Shi Q, Chuang TS, Markowitz D, Reisfeld RA, Luo Y (2011) Downregulation of transcription factor SOX2 in cancer stem cells suppresses growth and metastasis of lung cancer. Br J Cancer 104(9):1410–1417. https://doi.org/10.1038/bjc.2011.94CAS PubMed PubMed Central Google Scholar
- Liu K, Lin B, Zhao M, Yang X, Chen M, Gao A, Liu F, Que J, Lan X (2013) The multiple roles for Sox2 in stem cell maintenance and tumorigenesis. Cell Signal 25(5):1264–1271. https://doi.org/10.1016/j.cellsig.2013.02.013CAS PubMed Google Scholar
- Villodre ES, Kipper FC, Pereira MB, Lenz G (2016) Roles of OCT4 in tumorigenesis, cancer therapy resistance and prognosis. Cancer Treat Rev 51:1–9. https://doi.org/10.1016/j.ctrv.2016.10.003CAS PubMed Google Scholar
- Yoon TJ, Kim TJ, Lee H, Shin KS, Yun YP, Moon WK, Kim DW, Lee KH (2008) Anti-tumor metastatic activity of β-glucan purified from mutated Saccharomyces cerevisiae. Int Immunopharmacol 8(1):36–42CAS PubMed Google Scholar
- Queiroz EA, Fortes ZB, da Cunha MA, Barbosa AM, Khaper N, Dekker RF (2015) Antiproliferative and pro-apoptotic effects of three fungal exocellular β-glucans in MCF-7 breast cancer cells is mediated by oxidative stress, AMP-activated protein kinase (AMPK) and the Forkhead transcription factor, FOXO3a. Int J Biochem Cell Biol 67:14–24CAS PubMed Google Scholar
- Kobayashi H, Yoshida R, Kanada Y, Fukuda Y, Yagyu T, Inagaki K, Kondo T, Kurita N, Suzuki M, Kanayama N (2005) Suppressing effects of daily oral supplementation of beta-glucan extracted from Agaricus blazei Murill on spontaneous and peritoneal disseminated metastasis in mouse model. J Cancer Res Clin Oncol 131(8):527–538CAS PubMed Google Scholar
References
