A critical review on production and industrial applications of beta-glucans
Highlights
- •Beta-glucan has been increasingly used by the food and other industries.
- •Latest progress on production and application of glucans was summarized.
- •Physicochemical properties and chemical modifications of β-glucan were reported.
- •Potential value-added products derived from β-glucans were also summarized.
- •β-glucans will play an increasing role in future global food and medical sectors.
Abstract
A great interest of β-glucans with many health-promoting and prebiotic properties has been registered. β-Glucans are major bioactive compounds known to have biological activities including anti-cancer, anti-inflammatory, and immune-modulating properties. Due to the specific physical properties of β-glucan, such as water solubility, viscosity, and gelation, it has been increasingly used by the food and other industries. The aim of this review is to present an overview on the production technologies of β-glucan, such as extraction, isolation, purification technologies from different sources, for instance yeast, fungi, bacteria, and cereal, aiming its optimization for more effective production processes. Furthermore, the physicochemical properties, chemical modifications, possible industrial applications and future prospects of β-glucans in foods, medicines, cosmetics, and other potential value-added products are also summarized. Data indicate that β-glucans will play an increasing role in current and future global food and medical sectors.
Keywords
β-Glucan
Production process
Preparation technology
Physical properties
Extraction
Industrial application
Value-added products
Chemical compounds studied in this article
Beta-glucan (PubChem CID: 136779747)
Glucose (PubChem CID: 53782692)
Hydrochloric acid (PubChem CID: 24845468)
Ammonium sulfate (PubChem CID: 6097028)
2-Propanol (PubChem CID: 3776)
Ethanol (PubChem CID: 702)
Sodium hydroxide (PubChem CID: 24845831)
Potassium hydroxide (PubChem CID: 24844923)
Monochloroacetic acid (PubChem CID: 300)
Congo red (PubChem CID: 11313)
1. Introduction
β-Glucans are polysaccharides of d-glucose monomers linked through β-glycosidic bonds. As a kind of dietary fiber (DF), β-glucan could be found in a variety of natural sources such as yeast, mushrooms, bacteria, algae, barley and oat (Zhu, Du, Bian, & Xu, 2015). β-Glucan exhibits a broad spectrum of biological activities including anti-tumor, immune-modulating (Rieder & Samuelsen, 2012), anti-aging and anti-inflammatory properties. β-Glucans have attracted attention over the years because of their physical and chemical properties. β-Glucan from different sources and with different molecular weights has different biological activities (Du & Xu, 2014). β-Glucan from baker’s yeast consists of β-(1 → 3) and (1 → 6) linkages (Fig. 1(a)). However, other β-glucans, derived from cereals, are polysaccharides of glucose residues with β-(1 → 3) and β-(1 → 4) linkages (Fig. 1(b)). And cellulose is a (1 → 4) β-glucan; curdlan is a (1 → 3) β-glucan; β-glucan from lichen may contain either (1 → 3) (1 → 4) or (1 → 3) (1 → 6)-β-glucans. Fungal β-glucan has shown effectiveness as an immune system booster and an anti-tumor substance (Du, Lin, Bian, & Xu, 2015). β-Glucan from cereals help to lower cholesterol and blood glucose (Zhu et al., 2015).
 Fig. 1
Fig. 1Du et al. (2015) reviewed the anti-inflammatory effects of fungal β-glucans. Du, Bian, and Xu (2014) also discussed skin health promotion effects of natural β-glucan derived from cereals and microorganisms. However, the review on production, physical properties and industrial applications of β-glucan has received little attention. The purpose of this paper is to provide an overview based on the published data (including papers and US patents on production, extraction and purification of β-glucan from various sources (Table 1). We also presented the physical properties of β-glucan. Finally, we included the potential applications in foods, cosmetics, pharmaceutical industry and other health products applications of β-glucan.
Table 1. US Patents documented on β-glucans.
| Patent name | Patent number | Field of Patent | References |
|---|---|---|---|
| Production of beta-glucan, bran, protein, oil and maltose syrup from waxy barley | 4804545 | Production technology | Georing & Eslick, 1988 |
| Beta-glucan | 4769363 | Production technology | Misaki, Sone, Yoshida, & Takeuchi, 1988 |
| Methods of purifying beta glucans | 7550584 B2 | Production technology | Bahl, Vercellotti, Vercellotti, & Klein, 2009 |
| Method of using beta-glucan from Schizophyllum commune | 0023681 A1 | Production technology | Kim, Park, & Lee, 2009 |
| Particulate-soluble glucan preparation | 20130237497 A1 | Production technology | Cox, 2013 |
| Substantially purified beta (1,3) finely ground yeast cell wall glucan composition with dermatological and nutritional uses | 5576015 | Production technology | Donzis, 1996 |
| Methods for extracting cereal β-glucans | 5518710 | Production technology | Bhatty, 1996 |
| Glucan preparation | 5622939 | Production technology | Jamas, Easson, & Ostroff, 1997 |
| Method for producing soluble glucans | 5811542 | Production technology | Jamas, Easson, & Ostroff, 1998 |
| Composition for external application containing a beta-1,6-branched-beta-1,3-glucan | 0029253 A1 | Production technology | Park et al., 2001 |
| β-Glucan products and extraction process from cereals | 0192770 A1 | Production technology | Morgan, 2002 |
| Method for concentrating β-glucan | 6624300 B2 | Production technology | Potter, Fisher, Hash, & Neidt, 2003 |
| Beta-glucan compositions and process therefore | 6835558 B2 | Production technology | Van Lengerich, Gruess, & Meuser, 2004 |
| Beta-1,3-1,6-D-glucan and its use | 0271613 A1 | Production technology | Suzuki, Nakamura, Nakayama, & Nishikawa, 2005 |
| Extraction and purification method for cereal beta-glucan | 012149 A1 | Production technology | Redmond & Fielder, 2006 |
| Process for extraction of β-glucan from cereals and products obtained therefrom | 7138519 B2 | Production technology | Morgan, 2006 |
| Preparation method of beta-glucan from Schizophyllum commune and composition for experimental application comprising the same | 0160043 A1 | Production technology | Kim, Park, & Lee, 2008 |
| Personal care compositions comprising alpha-glucans and/or beta-glucans | 0095731 A1 | Application in health products | Mitra, 2008 |
| Novel therapeutic uses of glucan | 0293278 A1 | Application in medicine | Kelly, 2006 |
| Soluble phosphorylated glucan: Methods and compositions for treatment neoplastic diseases | 4818752 | Application in medicine | Williams, Browder, DiLuzio, & DiLuzio, 1989 |
| Soluble phosphorylated glucan: Methods and compositions for wound healing | 4975421 | Application in medicine | Williams, Browder, & DiLuzio, 1990 |
| Glucan compositions | 0156563 A1 | Application in cosmetics | Baschong, Monglat, & Ochs, 2009 |
| Beta-glucan process, additive and food product | 6749885 B2 | Application in food | Cahill, Fenske, Freeland, & Hartwig, 2003 |
| Therapy-enhancing glucan | 20130230535 A1 | Application in medicine | Cheung, 2013 |
| Composite glucan and method for preparing the same | 20140031542 A1 | Application in cosmetics | Chen, 2014 |
2. The production of β-glucans from different sources
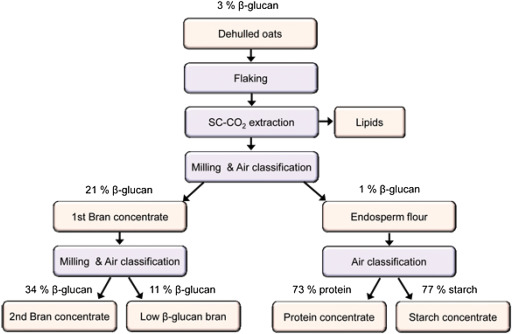
The production of fungal β-glucan is accompanied by a remarkable increase in broth viscosity because of both biopolymer accumulation and microbial growth (Garcia-Ochoa, Gomez Castro, & Santos, 2000). A critical point affecting the technical feasibility of fungal β-glucan production is represented by the potential interferences of the β-glucan released during the fermentation (Crognale, Bruno, Fidaleo, Moresi, & Petruccioli, 2007). A range of extraction and purification methods are available for the production of cereal β-glucans. The nature of extraction procedure has a profound effect on the structure and molecular weight of β-glucan (Zhu et al., 2015). The differences in β-glucan molecular weight estimates might come from the methods used for extraction and purification, aggregation phenomena and depolymerization events occurring during the extraction step (Lazaridou & Biliaderis, 2007). To determine the molecular-structural characteristics of isolated β-glucans, the extraction procedures need to retain the integrity of β-glucan molecules and to optimize the β-glucan yield and purity (Brennan & Cleary, 2005). The most appropriate extraction method depends on the sources and the structures of β-glucans. Hot water extraction is the most common extraction method of β-glucan. Other extraction methods include alkali extraction (Kao et al., 2012), ultrasound-assisted extraction (Du, Zhu, & Xu, 2014a), microwave-assisted extraction (Ookushi, Sakamoto, & Azuma, 2006), enzyme as elicitors extraction (Park, Ka, & Ryu, 2014) and acidic extraction (Park et al., 2001). Some of existing extraction methods have drawbacks such as a long extraction time (Routray & Orsat, 2012), high process costs, and reduced environmental sustainability. Some of the new extraction methods such as accelerated solvent extraction (Du et al., 2014a) and superheated water extraction (Adachi, Kowhakul, Masamoto, & Shigematsu, 2013) are useful in mediating associated extraction problems along with increased yields. The whole extraction and production process of β-glucan in lab scale and pilot scale can be summarized as various steps as shown in Fig. 2. The industrial scale of production process of β-glucan was shown in Fig. 3. Table 2 presents the β-glucan production yields reported in the literature, obtained in different production studies.
 Fig. 2
Fig. 2 Fig. 3
Fig. 3Table 2. β-glucans yields and production technologies of β-glucan from various sources.
| Sources | Production process | Production time | Production yield (%) | Reference |
|---|---|---|---|---|
| Hull-less barley | Remove starch and protein, accelerated solvent extraction and precipitate with anhydrous ethanol | 9 min | 16.39 | Du et al., 2014a |
| Hull-less barley | Dry-milled, water extract and freeze-dried | – | 6.3–15.4 | Bhatty, 1992 |
| Hulled barley | The high temperature high moisture extrudation | – | 8 | Sharma & Gujral, 2013 |
| Barley | Extraction with water, centrifugation, precipitation with ethanol and homogenization | 30 min | 5.54 | Temelli, 1997 |
| Barley | Sequential treatment with water at 40 °C, water at 90 °C with heat-resistant alpha -amylase and 1 M NaOH at room temperature | – | 2.5 | Saulnier, Gévaudan, & Thibault, 1994 |
| Barley | Water extraction, enzymatic removal of starch and protein and subsequent precipitation with ammonium sulfate saturation. | 2 h | 5.93 | Irakli, Biliaderis, Izydorczyk, & Papadoyannis, 2004 |
| Barley | Hot water Extraction, high speed stirring and centrifuge | 90 min | 5.4 | Ahmad, Anjum, Zahoor, Nawaz, & Din, 2009 |
| Oat | Precipitate with methanol, ethanol and isopropanil, coagulate and filtration | – | 0.4 | Redmond & Fielder, 2006 |
| Oat | Enzymes extraction | 6 h | 5.14 | Ahmad, Anjum, Zahoor, Nawaz, & Ahmed, 2010 |
| Oat | Enzymes and controlled temperature extraction, centrifugation and freeze dry. | – | 76 | Immerstrand et al., 2009 |
| Rolled oats | Alkaline treatment, isoelectric precipitation and alcohol precipitation | – | 5.24 | Dawkins & Nnanna, 1993 |
| Oat bran | Alkaline treatment, isoelectric precipitation and alcohol precipitation | – | 6.28 | Dawkins & Nnanna, 1993 |
| Wheat | Alkaline extraction, Acid and enzymatic treatment | – | – | Cui, Wood, Blackwell, & Nikiforuk, 2000 |
| Wheat | Fast extraction, decolourisation, filtration, drying and β-glucan yield weighing | – | 0.4–1.4 | Hozova, Kuniak, Morvcikova, & Gajdosova, 2007 |
| Rye | Air-dried, ground, enzyme hydrolysis and β-glucan determination | – | 0.7–2.4 | Genç, Ozdemir, & Demirbas, 2001 |
| Schizophyllum commune | Seed culture preparation, optimization of fermentation medium and schizophyllan production | 168 h | 0.8 | Kumari, Survase, & Singhal, 2008 |
| Ganoderma lucidum | Extraction using dilute NaOH solution and Sephadex G-15 gel-filtration chromatography | – | 18 | Kao et al., 2012 |
| Agaricus brasiliensis | Sequentially extracted with 350 ml water, concentrated, dialyzed and DEAE-cellulose column chromatography | – | 4.3 | Camelini et al., 2005 |
| Astraeus hygrometricus | Aqueous extraction, DEAE cellulose bag and Sepharose 6B column | – | – | Chakraborty, Mondal, Pramanik, Rout, & Islam, 2004 |
| Termitomyces eurhizus | Hot alkaline extraction, centrifugation, DEAE cellulose bag and freeze dry | – | – | Chakraborty, Mondal, Rout, & Islam, 2006 |
| Flammulina velutipes | Successive hot extraction with water and KOH and submitted to freeze-drying | – | 3.7 | Smiderle et al., 2006 |
| Boletus erythropus | Water extraction, centrifugation, DEAE Trisacryl M column and S 400 HR column | – | – | Chauveau, Talaga, Wieruszeski, Strecker, & Chavant, 1996 |
| Paenibacillus polymyxa | Seed culture was supplemented with carbon source to induce glucan production | 3 days | 1.06 | Jung et al., 2007 |
| Botryosphaeria rhodina | β-Glucan production were monitored in a stirred-tank bioreactor | 78 h | 1.97 | Crognale, Bruno, Fidaleo, Moresi, & Petruccioli, 2007 |
| Yeast | Alkaline extraction, DEAE-cellulose and ConA chromatography | – | 4 | Lee et al., 2001 |
| Brewer’s yeast | alkaline extraction, | 1 h | 51 | Suphantharika, Khunrae, Thanardkit, & Verduyn, 2003 |
| Agrobacterium sp. ATCC 31750 | Two-step culture, centrifugation, washing with distilled water | 120 h | 6.6 | Kalyanasundaram, Doble, & Gummadi, 2012 |
| Agaricus bisporus | Ultrasonic-assisted extraction, precipitation with ethanol, centrifugation | 62 min | 6.02 | Tian et al., 2012 |
2.1. β-Glucan from cereals
Cereal β-glucans are distinctive polymers of glucose differentiated from other polymers not only by their source but by their physicochemical properties (Redmond & Fielder, 2006). The extraction of cereal β-glucan is very difficult, it make cereal β-glucan becomes the more expensive than β-glucans from other sources (Daou & Zhang, 2012). The process of production of cereal β-glucan has continued to be developed over the years. The extraction methodologies are based on the solubility of β-glucan in hot water and in alkaline solutions, separation of the dissolved proteins by isoelectric precipitation, and precipitation of the β-glucan by ammonium sulfate, 2-propanol, or ethanol (Wood, Siddiqui, & Paton, 1978). For research purposes, repeated precipitations and enzymatic hydrolysis of residual starch are used in further purification, and a purity of 99% has been reached (Westerlund et al., 1993, Wood et al., 1991).
Du et al. (2014a) developed extraction method of β-glucan from hull-less barley bran using water by accelerated solvent extraction (ASE) combined with response surface methodology. The ASE technique, ultrasound assisted extraction (UAE), microwave-assisted extraction (MAE), and reflux extraction was also compared. The extraction yield of β-glucan with ASE method was 16.39 ± 0.3%. ASE produced much higher β-glucan than other methods used and was found to be more environmentally friendly extraction, less extraction discrimination and shorter time, and could be useful to the development of industrial extraction processes. Furthermore, Redmond and Fielder (2006) conducted the extraction and purification of oat β-glucan. The high purity of β-glucan obtained allows for the preparation of clear, colorless viscous liquid preparations. These preparations are stable in gelling effects when kept at ambient temperatures and low ash concentrations.
A process for producing β-glucan from barley was established using water as extracting solvent. This process for recovering β-glucan from an aqueous solution of β-glucan comprising freezing the solution, allowing the solution to thaw and separating solids from the resultant suspension (Morgan, 2002). Morgan (2006) studied the process for obtaining β-glucan product from barley and oats. The process includes the steps of forming flour from the cereal grain, mixing the flour with water to form a slurry of an aqueous solution of β-glucan and a solid residue, separating the aqueous solution from the solid residue, and removing water from the aqueous solution by evaporation or ultrafiltration or combinations thereof to form a β-glucan containing gel or solid. Van Lengerich, Gruess, and Meuser (2004) suggested a novel method for producing high quality β-glucan enriched soluble DF products. An aqueous extraction slurry of β-glucan-containing grain material is homogenized, acidified and enzymatically digested to reduce viscosity and optimize separation of insolubles from the aqueous extract solution. The resulting extract is heat-processed to precipitate the denatured protein components and subsequently processed to provide the dry products.
Bhatty (1996) investigated a method for producing high levels of β-glucan from cereals using sodium hydroxide as an initial extraction solvent. The extract can be further purified to render a β-glucan preparation. Potter, Fisher, Hash, and Neidt (2003) produced β-glucan from milled cereal bran, grain and distiller’s dried grain. This process include providing an alkaline aqueous extract of a β-glucan source; acidifying or neutralizing the extract and heating the extract to about 60 °C–100 °C; cooling the extract, whereby a flocculate is formed; acidifying the cooled extract if the extract was neutralized; and removing the flocculate from the aqueous solution to form an intermediate solution. Beer, Arrigoni, and Amado (1996) produced large amounts of good quality oat gum rich in (1 → 3) (1 → 4)-β–D-glucan using different processing technologies. Untreated and enzyme-deactivated oat bran concentrate were extracted with aqueous sodium carbonate at pH 10 and 40 °C. Oat gums were subsequently isolated either by dialysis, ultrafiltration, or alcoholic precipitation on small, medium, and pilot plant scales. It was possible to produce oat gums with a β-glucan content of 60–65% with all three methodologies. The results showed that alcoholic precipitation would be the process of choice, but ultrafiltration and dialysis are useful alternatives to produce large amounts of oat gum rich in β–D-glucan.
Sharma and Gujral (2013) evaluated the effect of extrusion variables (temperature and moisture) on hulled barley β-glucan and physicochemical properties. The β-glucan extractability was increased by up to 8% after extrusion with extrudates from the high temperature and high moisture. Oat β-glucans were extracted from milled seeds of two Greek cultivars and partially purified by pH adjustment of the β-glucan solutions to 4.5. Chemical analysis of the extracted gums revealed that they were composed mainly of β-glucans (>85%). The fine structure of the β-glucan preparations was also assessed (Skendi, Biliaderis, Lazaridou, & Izydorczyk, 2003). In another study, Irakli, Biliaderis, Izydorczyk, and Papadoyannis (2004) isolated β-glucans from six Greek barley cultivars using water extraction, enzymatic removal of starch and protein and subsequent precipitation with ammonium sulfate saturation, the purity of barley β-glucans was high (>93% on dry weight basis). Vasanthan and Temelli (2001) used an organic solvent and water, acidified water and aqueous alkali as a solvent for the slurrying of a grain flour. The process is particularly effective in concentrating beta-glucans in a state close to its native form from the endosperm of barley and oat grains (Vasanthan & Temelli, 2008.).
β-Glucans in oats are linear homopolysaccharides of d-glucopyranosyl units. The molecular-structural characteristics of β-glucans in oats, which distinguishes them from cereal β-glucans of different botanical origins, include the ratio of tri-to tetramers, amount of cellulose oligomers, ratio of β-(1 → 4)/–(1 → 3) linkages, and molecular weight (Cui, Wood, Blackwell, & Nikiforuk, 2000).
2.2. β-Glucan from the fruiting body of fungi
Extraction in hot or boiling water is the most common and convenient method for extracting water-soluble fungal polysaccharides (Yan, Wang, & Wu, 2014). Bhanja et al. (2014) extracted and isolated two water-insoluble glucans from the fruiting bodies of mushroom Ramaria botrytis. On the basis of chemical and NMR studies, the structure of the linear (1 → 3)-α–D-glucan and non-linear β-(1 → 3)-D-glucan branched at O-6 were established. Lentinan was extracted and isolated from the fruiting body of Lentinus edodes using fractional precipitation with ethanol, fractional solubilization with acetic acid, and diethylaminoethyl cellulose column chromatography (Chihara, 2001). Kim, Kim, Choi, and Lee (2005) extracted β-glucan from the fruiting bodies of Agaricus blazei using hot water with 3 h. The crude β-glucan extracts were applied to Sephadex G-50 column and Sephadex A-25 column for purification. Kumari, Survase, and Singhal (2008) optimized the schizophyllan production from the fruiting body of Schizophyllum commune using one factor at a time method and response surface method. The maximum production of schizophyllan was attained from an initial value of 1.06 g L−1 to 8.06 g L−1. Moreover, Kao et al. (2012) had successfully isolated β-glucan, in high yields, from the waste residue of fruiting bodies of Ganoderma lucidum. They purified the β-glucan by Sephadex G-15 gel-filtration chromatography. Camelini et al. (2005) extracted β-glucan from basidiomycete Agaricus brasiliensis in different maturity stages of fruiting body with boiling water, and then the crude polysaccharide was passed through a DEAE-cellulose column chromatography for further purification. The yields of β-glucans increased from 42 mg g−1 fruiting bodies (dry weight) in immature stage to 43 mg g−1 in mature stage with immature spores. Smiderle et al. (2006) obtained the crude β-glucan from the dried mushroom Flammulina velutipes, and then residue was subjected to further alkaline extraction at 100 °C, using 2% and then 25% KOH. After centrifugation of the fractions, insoluble β-glucan (3.7% yield) was isolated.
Sen et al. (2013) also extracted β-glucan from the fruiting bodies of a hybrid mushroom, pfls1h of Pleurotus florida and Lentinus squarrosulus (Mont.) Singer using distilled water. The crude polysaccharide was passed through Sepharose 6B gel permeation chromatography using water as the eluent with a flow rate of 0.5 mL min−1. Two fractions of purified polysaccharide were obtained. Liu et al. (2014) obtained a purified β-glucan by precipitating a hot-water extract from fruiting bodies of Ganoderma lucidum with 20% (V/V) ethanol. The total carbohydrate content was 95.9% in prepared β-glucan. Tian et al. (2012) optimized the ultrasonic-assisted extraction of polysaccharides from Agaricus bisporus using the central composite design. The highest yield of 6.02% was achieved under the optimal extraction conditions.
The (1 → 3, 1 → 6)-β-glucans play the role of structural frame for the fungal cell and define its shape and rigidity. Some (1 → 3, 1 → 6)-β-glucans can also serve as fungal storage carbohydrates (Zeković, Kwiatkowski, Vrvić, Jakovljević, & Moran, 2005).
2.3. β-Glucan from the mycelium of fungi
Kim, Park, and Lee (2009) provided a method for mass production of β-glucan from S. commune, comprising subjecting mycelia of S. commune to liquid culture with an addition of a synthetic adsorbent. Kim, Kang, and Ro (2013) demonstrated generation of high β-glucan producing mutant strains of Sparassis crispa, additional culture optimization further increased β-glucan productivity of the mutant strains. Recently, Park et al. (2014) enhanced the β-glucan content in the sawdust-based cultivation of cauliflower mushroom (Sparassis latifolia) using three kinds of enzymes (chitinase, β-glucuronidase, and lysing enzyme complex) as elicitors. Several enzyme treatments resulted in an increase in β-glucan concentration by up to 31% as compared to that of the control. These findings concluded that the stimulation of fungal cell wall by biotic elicitors may enhance the β-glucan concentration in cauliflower mushrooms.
2.4. β-Glucan from yeast
Donzis (1996) purified β-glucan derived from finely ground yeast cell wall and explored nutritional and dermatological applications. Lee et al. (2001) purified a soluble β-glucan from the cell wall of yeast. β-Glucan was first rendered soluble form from the yeast cell wall by alkaline extraction. The extract was purified using DEAE-cellulose and ConA chromatography. β-Glucan thus prepared was completed free of mannoprotein and was soluble at neutral pH. Zlatkovic, Jakovljevic, Zekovic, and Miroslav (2003) isolated a β-glucan from active dry baker’s yeast. The main structural feature of the polysaccharide deduced on the basis of the obtained results is a linear chain of (1 → 3)-linked β–D-glucopyranoses, a part of which is substituted through the positions O-6. Suphantharika, Khunrae, Thanardkit, and Verduyn (2003) prepared β-glucan from insoluble spent brewer’s yeast cell wall. A simple alkaline extraction was applied and optimized. The results indicated that the optimum extraction parameters were as follow: extraction time for 1 h, NaOH concentration for 1.0 N, extraction temperature for 90 °C and a yeast cell wall to NaOH solution ratio of 1:5 (w/v), the yield was 51%. Additionally, Thammakiti, Suphantharika, Phaesuwan, and Verduyn (2004) prepared β-glucan obtained from spent brewer’s yeast for potential food applications. The production processing was as follows: The brewer’s yeast was autolysed and the cell walls were homogenized, extracted firstly with alkali, then with acid, and then spray-dried.
The yeast and fungal glucans share a common structure: Primary backbone chains of (1 → 3)-linked β-glucopyranosyl units, along which are randomly dispersed side chains of β–d-glucopyranosyl units attached by (1 → 6) linkages (Zlatkovic et al., 2003). These (1 → 3, 1 → 6)-β-glucans are usually highly branched; often present as an inner wall layer and are sometimes covalently associated with other cell wall polymers.
2.5. β-Glucan from bacteria
Microbial β-glucans are produced through multi-stage processes that need mycelium growth, substrate consumption and catalyzation of glucan-hydrolytic enzymes in shaken flasks and bioreactor. Stack, Kearney, Stanton, Fitzgerald, and Ross (2010) investigated β-glucan production from Lactobacillus paracasei NFBC 338 using membrane-associated glycosyltransferase enzyme (encoded by the gtf gene). The results indicated that production of a β-glucan exopolysaccharide by strains destined for use as probiotics may afford them greater performance during cultivation, processing, and ingestion. As such, expression of the gtf gene may prove to be a straightforward approach to improve strains that might otherwise prove sensitive in such applications. Moreover, Jung et al. (2007) developed a β-glucan from 4 kinds of Paenibacillus polymyxa isolated from Korean soil. The results showed that a novel strain JB115 had the highest yield β-glucan 10.6 ± 2.3 g L−1. The β-glucan was proved to be a β-(1 → 3) – and β-(1 → 6) – linked glucan structure using FT-IR, 1H NMR, and 13C NMR spectra. Additionally, Crognale et al. (2007) produced β-glucan from Botryosphaeria rhodina DABAC-P82 by detecting simultaneously glucan-hydrolytic enzymes and their localization, culture medium rheology and oxygen transfer. They concluded that the greatest β-glucan accumulation in the bioreactor was found to be associated with nitrogen and dissolved oxygen concentrations. Kalyanasundaram, Doble, and Gummadi (2012) studied the production of curdlan by Agrobacterium sp. ATCC 31750 in shake flask cultures and bioreactor. They used two-stage culture technique to optimize favorable pH value for curdlan production in the mutant strain. The results indicated that with increase in pH the cells consumed more sucrose and produce more curdlan. Sato et al. (2012) prepared a water-soluble β-glucan with low molecular weight from Aureobasidium pullulans 1A1, alkaline extraction and ethanol precipitation.
2.6. β-Glucan-binding protein
β-Glucan generally is found to consist of repeated glucose subunits joined together by a beta linkage between different carbons within the glucose ring. In nature they are usually found together bound in a sugar–protein complex. The β-glucan-binding proteins are a heterogeneous group of proteins with variations in size, glucan-binding domain, glucan-binding affinity, distribution, and function. The protein component is necessary for the potent activity of the complex (Kawagishi, Kanao, Inagaki, & Mizuno, 1990). Mithöfer, Lottspeich, and Ebel (1996) obtained a low abundance β-glucan-binding protein from soybean using a rapid, simple and one-step purification method. The results showed that the affinity-based purification technique was more efficient than conventional methods. Additionally, Mohanty et al. (2015) purified and biochemically characterized a β-glucan-binding protein from the haemolymph of fresh water prawn Macrobrachium rosenbergii with heparin affinity chromatography.
2.7. β-Glucan from other sources
Watanabe et al. (2013) demonstrated β-glucan paramylon from Euglena and the antitumor activity of β-glucan against preneoplastic colonic aberrant crypt foci in mice. It is considered that β-glucans, such as paramylon and its isomer amorphous paramylon, have preventive effects against colon cancer and are more effective against the condition than Euglena. Yoshida, Honda, Tsujimoto, Uyama, and Azuma (2014) extracted 6.6% β-glucan from corn pericarp with NaOH solution and initially fractionated into neutral and acidic parts, and then isolated β-glucan from corn pericarp hemicelluloses using a combination of anion-exchange chromatography and affinity chromatography on a cellulose column. Min et al. (2012) isolated β-glucan from six kinds of Korean commercial Makgeolli (an alcoholic beverage native to Korea). They identify R-12 commercial raw Makgeolli as a high content of immuno-stimulating β-glucan. Vetvicka et al. (2007) extracted and purified β-glucan phycarine from the marine brown algae Laminaria digitata using hot water and two steps ultrafiltrations. One study was undertaken to use a macchinetta extractor to obtain β-glucan from Japanese cedar bark with superheated water. Autoclave extraction was effective for producing a high extraction yield (Adachi et al., 2013). Extraction and purification of β-glucan is a tedious process involving numerous steps of liquid and solid phase separations. More studies may be needed to confirm the importance of β-glucan production methods.
3. Chemical modification of β-glucan
β-Glucan is an important bioactive compound for human health, but its low solubility has led to the development of chemical modification technologies to improve bioavailability. Several methods to modify β-glucan are laid out to improve their functional and technological properties via physical and chemical cross-linking reactions (Ahmad, Mustafa, & Che Man, 2015). In this respect, β-glucans can be chemically modified to obtain various derivatives with potential industrial or medicinal importance (Synytsya & Novák, 2013). Because of the insoluble chemical nature of β-glucan, particulate β-glucans are not suitable for many medical applications (Zeković et al., 2005). Various approaches in changing or modifying the chemical structures of β-glucan and transforming it to a soluble form have been reported. Modifications will affect the bioactive properties of β-glucan either positively or negatively. Carboxymethyl glucan (CM glucan) (Fig. 1(c)) is a water soluble polysaccharide modified from the insoluble β-glucan by carboxymethylation. The structure of the CM glucans in the solution depends upon the degree of substitution and with the increase of the degrees of substitution, a transition from triple-helical structure through single helical to random structure takes place (Kogan, 2000). CM glucan has biological activities which are consistent with its insoluble form. The improvement of solubility enables its application as a biomaterial for pharmaceutical and cosmetic aspects (Kanlayavattanakul & Lourith, 2008). The soluble phosphorylated glucans derived from the yeast Saccharomyces cerevisiae were investigated. This glucan is useful for promoting the wound healing process (Williams, Browder, & DiLuzio, 1990) and for prophylactic and therapeutic applications against neoplastic, bacteria, viral, fungal and parasitic diseases (Williams, Browder, DiLuzio, & DiLuzio, 1989).
Owing to β-glucan derived from Poria cocos hardly exhibits bioactivities. To extend its use, Wang et al. (2014) synthesized three types of (1 → 3)-β–D-glucan derivatives, which were sulfated (1 → 3)-β–D-glucan (Fig. 1(d)), carboxymethyl (1 → 3)-β–D-glucan and carboxylmethyl (1 → 3)-β–D-glucan sulfate. These results showed that multiple modifications of polysaccharides may bring the derivatives with excellent properties and various applications. In the homogenous sulfating of β-glucan from Saccharomyces cerevisiae, the 125 kDa fraction of the sulfated material accounted for only 1% of the product, with 99% of molecular weight of sulfated glucan being 14.5 kDa. The 13C NMR confirmed the presence of (1 → 3)-β interchain linkage in the polysaccharide chain and in solution the chains were self-associated in a triple helix form (Williams et al., 1992). Some chemically modified β-glucans from Volvariella volvacea were obtained and the inhibitory activity on the growth of mouse-transplanted tumors was studied (Kishida, Sone, & Misaki, 1992). Conversion of the glucosyl groups substituted at O-6 atoms of the (1 → 3)-linked d-glucose residues into the corresponding polyhydroxyl groups gave significant enhancement of the original activities both on allogeneic and syngeneic tumors. Conversion of the epoxy groups to hydrophilic glycerol groups remarkably enhanced the antitumor activity (Kishida et al., 1992). In another study, Berdal et al. (2007) prepared an aminated β-glucan (Fig. 1(e)), a water-soluble derivative of curdlan. They found this aminated β–D-glucan could improve wound healing in an animal model with diabetes mellitus.
β-Glucans derived from oats were subjected to chemical modification, specifically 2, 2, 6, 6-tetramethyl-1-piperidine oxoammonium ion-mediated oxidation whereby C6 primary hydroxyl groups were selectively oxidized into carboxyl groups. The results showed that the oxidation increased water solubility of oat β-glucans. In this study, oxidized oat β-glucans have the potential as an active cholesterol-lowering ingredient (Park, Bae, Lee, & Lee, 2009).
Ding, Wang, Xiong, Zhao, and Huang (2013) optimized the carboxymethylation of β-glucan by a two-step alkalization and etherification with monochloroacetic acid. The effect of ball milling pretreatment on the original β-glucan and its carboxymethyl derivative were studied on the basis of optimized technology for the carboxymethylation of β-glucan from S. cerevisiae. As a result, the ball milling pretreatment was beneficial for original β-glucan to carboxymethylation. Therefore, the expanded use of modified β-glucans may be taken into account for better health improvement.
4. Effects of physical and chemical properties on biological activities of β-glucans
Studies have been conducted to investigate the physical properties of β-glucans. Temelli (1997) investigated the physical properties, such as viscosity, whippability, foam stability and emulsion stabilizing capacity of the barley β-glucan gum to assess potential food applications. The results showed that maximum emulsion stability and viscosity were achieved at pH 7.0 and 55 °C. Viscosity increased with pH at constant temperature. Barley β-glucan gum shows a great potential as a thickener or stabilizer in products such as soups, sauces, desserts and salad dressings. Ahmad (2014) assessed the role of particle size of barley β-glucan concentrate (BGC) on two fundamental rheological properties namely oscillatory rheology and creep in a dough system. The results showed that all those information could be helpful to identify the particle size range of BGC that could be useful to produce a β-glucan enriched designed food. Moreover, Brummer et al. (2014) investigated that the textural and rheological properties of oat β-glucan gels with varying molecular weight compositions. The obtained results showed that the textural properties and melting profiles of β-glucan gels can be manipulated by adjusting the ratios of molecular weight fractions or addition of sugar for different applications. Liu (2010) evaluated the effects molecular weight and structure of β-glucan on viscosities of oat-flour slurries, the impacts of processing (steaming and flaking) on the viscosities and in vitro bile acid binding of oat-flour pastes were also discussed. The results showed that the greater bile acid binding capacity might have been caused by the β-glucan structural-molecular changes and/or the improved capacity of β-glucans through increasing availability during processing. The viscosity of the β-glucan solutions depends on their concentration, molecular weight, and structural features (Wood et al., 1994). For the structural characteristics of β-glucans, it was suggested that the amount of DP ≥ 5, ratio of DP3/DP4, and ratio of β-(1 → 3)/–(1 → 4) linkages are the important determinants of viscosity and solubility (Skendi et al., 2003). Irakli et al. (2004) explored the rheological behaviors of aqueous dispersions of β-glucans, particularly as it pertains to their gelation potential and the effect of added polyols on the textural properties of mixed gels made with these polysaccharides. These findings showed that β-glucans exhibited large differences in their flow behavior, shear thinning ability and gelling capacity.
The extraction needs careful attention as extraction process may affect the physiochemical properties of extracted β-glucan. Generally, extracted pellets had good water retention and foaming capacity (Ahmad, Anjum, Zahoor, Nawaz, & Din, 2009). Some authors reported the various physicochemical properties of barley β-glucan as affected by different extraction procedures. A significant variation in extraction methods was observed with respect to water binding capacity and foaming capacity. A significant impact of extraction methods was also observed on color parameters of β-glucan. A negative correlation exists between foaming capacity and viscosity, whereas a positive correlation observed between soluble fiber and viscosity. Thammakiti et al. (2004) investigated the effects of the homogenization on the chemical composition, rheological properties and functional properties of β-glucan from baker’s yeast. Homogenized yeast cell walls exhibited higher β-glucan content and apparent viscosity than those which had not been homogenized because of fragmentation of the cell walls and higher release of β-glucan. When compared with commercial β-glucan from baker’s yeast, it was found that the β-glucan obtained through homogenization had higher apparent viscosity, water-holding capacity and emulsion stabilizing capacity, but very similar oil-binding capacity.
The molecular weight values of β-glucans in oats ranged from 0.35 × 105 to 29.6 × 105 (Yao, Jannink, & White, 2007). Moreover, based on NMR data and methylation analysis, the calculated ratios of β-(1 → 4)/–(1 → 3) linkages in oat β-glucans were within the range of 1.9–2.8 (Lazaridou, Biliaderis, & Izydorczyk, 2003).
The fine structure, molecular weight, conformation and solubility of β-glucan have been shown to influence biological activities (Bohn and BeMiller, 1995, Leung et al., 2006, Soltanian et al., 2009). β-Glucan molecular weight and fine structure, such as 1 → 3 to 1 → 6 linkage ratio, lengths, number and distribution of cellulosic oligosaccharides will together with amount and nature of co-extracted compounds in a β-glucan preparation influence solubility, aggregate formation and polymer conformation (Rieder & Samuelsen, 2012).
5. Industrial applications of β-glucans
β-Glucan has various physical properties such as thickening, stabilizing, emulsification, and gelation (Ahmad, Anjum, Zahoor, Nawaz, & Dilshad, 2012). β-Glucan has the potential to be used in acceptable health products that offer a wide range of added health benefits. There are some commercially available β-glucans products shown in Table 3. Much of the interest in the use of cereal β-glucans has stemmed from their use as a functional dietary fiber (Barsanti et al., 2011, Du et al., 2014b). β-Glucans have potential application in medicine and pharmacy, food, cosmetic and chemical industries, in veterinary medicine and feed production. The different industrial applications of β-glucans are summarized in Table 4.
Table 3. Commercially available β-glucans.
| Product name | Sources | Manufacturer | Molecular weight (kDa) |
|---|---|---|---|
| SymGlucan | Oat | Symrise Shanghai Co., Ltd. | 2.5 |
| Oat glucan | Oat | Shanghai Laibo Bio-chemical Co., Ltd. | 409 |
| Oat beta glucan | Oat | Kareber & Co GmbH Co., Ltd. | – |
| Glucan 300 | Baker’s yeast | Southeastern Pharmaceutical Cpmpnay | – |
| Biorigin | Baker’s yeast | Brazil Betamune Company | – |
| MacroGard | Baker’s yeast | Norway Biotec-Mackzymal Company | – |
| Immunowall | Baker’s yeast | Brazil Betamune Company | – |
| β-Glucan | Baker’s yeast | Sigma–Aldrich Company | – |
| Zymosan | Baker’s yeast | Sigma–Aldrich Company | – |
| Zymosan A | Yeast | BioParticles Company | – |
| Brewer’s yeast extract | Yeast | Cosfa International Trading Co., Ltd. | 0.2 |
| Yeast extract E100 | Yeast | Shanghai Youthretain Company | 0.2 |
| Natriance Brightener yeast extract | Yeast | Vincience Company | 0.04 |
| Carboxymethyl β-glucan C90 | Yeast | Angel yeast Co., Ltd. | 404 |
| Beta-glucan G70 | Yeast | Angel yeast Co., Ltd. | 1000 |
| Schizophyllum commune glucan | Schizophyllum commune | Bioland Technology Co., Ltd. | 664 |
| Ricetrim | Rice | VDF/FutureCeuticals, Inc. | – |
| Oattrime | Oat | VDF/FutureCeuticals, Inc. | – |
| Algamune | Algae | Algal Bioproducts | – |
Table 4. Industrial applications of β-glucans.
| Area of applications | Products | Functionality |
|---|---|---|
| Foods | Prebiotic sausage formulation with β-glucan | Noticeable effect on physical and sensory properties |
| Gluten-free bread with β-glucan | Acceptable results of sensory analysis | |
| Dairy products with β-glucan | Calorie-reduced and cholesterol-lowering | |
| Yogurts with β-glucan | Faster proteolysis, lower release of large peptides and a higher proportion of free amino acids | |
| Extruded ready-to-eat snacks | Manipulate the glycemic response | |
| Beverage containing β-glucan | Control food intake and reduce 24 h energy intake | |
| Cakes containing β-glucan | Good quality attributes | |
| Used in food products | As a thickening, water-holding, or oil-binding agent and emulsifying stabilizer | |
| Medicines | Wound dressing material | Larger inner cavity diameter |
| Transparent wound dressing sheet | Therapeutic efficacies comparable or superior to a commercial wound dressing | |
| Curing partial-thickness burns | Decrease post injury pain | |
| Curing burn-induced remote organ injury | Be effective against burn-induced oxidative tissue damage | |
| Poly-membranes containing β-glucan | Accelerated wound healing effects | |
| A bone-substituting material | Easy manipulation and good adaptation to the shape and dimensions of even large bone defects | |
| Vaccine delivery platform. | Can be exploited for vaccine development | |
| Cosmetics | Film-forming moisturizer | Efficacy for reducing fine-lines and wrinkles |
| Skin and dermatological compositions | Moisturization of skin or mucosa and anti-aging and revitalizing effect on the skin | |
| Cosmetic product | Defer skin aging, impart skin whitening effect and cure skin damage effectively | |
| Cosmetic product | Treat collagen loss in aging skin | |
| Cosmetic formulation containing CM β-glucan | With good spread ability and skin smoothing effect | |
| Emulsion containing CM β-glucan | Improve skin condition | |
| Cosmetic product containing yeast β-glucan | Enhance ulcer healing and increase epithelia hyperplasia | |
| Eye drops with mushroom β-glucan | Great moisture retention | |
| Feeds | Animal feed additive | Enhance immunity and as a potential antitumor agent |
| Fish feed additive | Increase the number of specific antibody secreting cells and specific Ig levels in serum | |
| Other health products | Materials for health foods | Useful |
| Personal care compositions containing β-glucan | Hair care actives | |
| Novel prebiotics | Health-promoting property | |
| β-Glucan nanoparticles | Exhibit antifungal activity against Pythium aphanidermatum | |
| Natraceutical product containing β-glucan | Useful |
5.1. Applications in foods
A kind of high levels of β-glucan was obtained from oat or barley grain. This product can be produced as an agglomerated food additive having at least about 18% β-glucan by dry weight. Methods are providing for enriching a product with the β-glucan agglomerated food additive (Cahill, Fenske, Freeland, & Hartwig, 2003). Sarteshnizia, Hosseinia, Bondarianzadeha, Colmenerob, and Khaksara (2015) optimized the prebiotic sausage formulation by resistant starch, β-glucan and starch according to D-optimal mixture design approach. β-Glucan had noticeable effect on physical and sensory properties of sausage. As a result, the production of prebiotic sausage using combination of β-glucan and resistant starch is possible.
Kittisuban, Ritthiruangdej, and Suphantharika (2014) analyzed the effects of hydroxypropylmethylcellulose, yeast β-glucan, and whey protein isolate on physical properties of gluten-free bread baked from formulas based on rice starch. As a consequence, the optimized rice starch bread formulated with yeast β-glucan was found to be acceptable according to the results of sensory analysis. Additionally, Sharafbafi, Tosh, Alexander, and Corredig (2014) incorporated high molecular weight oat β-glucan into milk to obtain calorie-reduced and cholesterol-lowering dairy products, and the phase behavior, rheological properties, and microstructure of this dairy product were analyzed. The results showed that the flow behavior of the mixtures with concentrations higher than the binodal curve was not only governed by the presence of β-glucan chains, but also by the formation of these structures. Additionally, Rinaldi, Rioux, Britten, and Turgeon (2015) found that yogurts contained with β-glucan and with pectin showed a faster proteolysis, a lower release of large peptides, and a higher proportion of free amino acids than those with starch or without β-glucan. Segregation between β-glucan and dairy proteins was hypothesized to explain faster proteolysis by concentration of enzymes and proteins in a separated phase. It has been suggested that β-glucan riched fractions from barley and mushroom used in the production of extruded ready-to-eat snacks. The results showed that the inclusion of these fractions could be utilized by the food industry to manipulate the glycemic response of extruded snack products (Brennan, Derbyshire, Tiwari, & Brennan, 2013). Barone Lumaga, Azzali, Fogliano, Scalfi, and Vitaglione (2012) evaluated the satiating capacity of three beverages containing barley β-glucan, or DF from fruit, or without DF. The results showed that a sucrose-sweetened beverage provided 3 g barley β-glucans can control food intake and it can even reduce 24 h energy intake. Kim et al. (2011) prepared the β-glucan-enriched materials from mushroom Lentinus edodes as a wheat flour substitute. That could be successfully used to produce cakes containing 1 g of β-glucan per serving with quality attributes similar to those of the control. Moreover, Lazaridou, Marinopoulou, Matsoukas, and Biliaderis (2014) evaluated the influence of flour particle size and hydrothermal treatment on β-glucan physicochemical properties and the potential nutritional functionality of barley rusks. The physiological impact of barley rusks was assessed by measuring the amount of solubilized β-glucans and the viscosity of the rusk extracts. Both autoclaving and increasing flour particle size significantly (p < 0.05) increased the viscosity of the rusk physiological extracts.
5.2. Applications in medicines
There is a latest review about clinical trials on the health beneficial effects of oral administration of β-glucan preparations (Samuelsen, Schrezenmeir, & Knutsen, 2014). β-Glucans have been used in several clinical trials to test their general effects on health and clarify the mechanisms responsible (Tungland & Meyer, 2002). Venkatachalam, Narayanan, and Doble (2013) used cyclic glucans (Fig. 1(f)) as wound dressing material due to cyclic glucans have larger inner cavity diameter. Moreover, Kofuji et al. (2010) obtained a transparent wound dressing sheet by forming a complex between β-glucan and chitosan. The β-glucan–chitosan complex sheets demonstrated therapeutic efficacies comparable or superior to a commercial wound dressing product by evaluating in wounds created on the dorsal surfaces of mice. The results indicated that β-glucan–chitosan complex sheet was a promising new wound dressing product. It is found that partial-thickness burns in children can be effectively treated with β-glucan with good results, even in infants and toddlers. β-Glucan markedly simplified wound care for the patient and family and seemed to significantly decrease post injury pain (Delatte, Evans, Hebra, & Adamson, 2001). β-Glucans merit consideration as therapeutic agents in the treatment of burn injuries (Toklu et al., 2006) and wound healing (Berdal et al., 2007). In one study, the putative protective effect of β-glucan treatment on burn-induced remote organ injury was investigated. The results indicated that administration of β-glucan were effective against burn-induced oxidative tissue damage in the rats (Toklu et al., 2006). In another study, Kim, Lee, Lee, Kwon, and Park (2012) investigated wound-healing effects of the poly(lactic-co-glycolic acid) membranes containing β-glucan, the findings showed that the membranes accelerated wound healing by improving the interaction, proliferation of cells, and angiogenesis. Therefore, these membranes can be useful as a skin substitute for enhancing wound healing. In another recent study by Sakurai, Mizu, and Shinkai (2001), the findings showed that the curdlan sulfates with DS from 1.7 to 8.7 mol% could form complexes with polycytidylic acid in the same manner as schizophyllan. The complex polynucleotide chain showed a significant resistance against enzymatic hydrolysis. It has been postulated that these water-soluble sulfated curdlan–nucleotide complexes could find application in gene technology in medicine.
Additionally, β-glucan has been proposed as a joining agent to combine granular ceramics into novel compact and elastic composite due to the unsatisfactory surgical handiness in implantable granular bioceramics. This study was to prepare a biphasic hydroxyapatite/glucan composite of elastic properties, which would allow for easy manipulation and good adaptation to the shape and dimensions of even large bone defects. The results confirmed that flexible hydroxyapatite/glucan composite had potential as a bone-substituting material (Belcarz et al., 2013). Moreover, Kogan et al. (2005) found that the radical scavenging activity of carboxymethylated β-glucan in the in vivo experiments. They observed a substantial decline in the level of plasmatic carbonyls. it was assumed that carboxymethylated β-glucan radical-scavenging properties could be responsible for the antioxidant activity in the adjuvant arthritis model. This brings the promise of possible medicinal applications of this glucan derivative in arthritis treatment.
Furthermore, a major challenge in vaccinology is the development of platforms and adjuvants that effectively promote protective T cell and antibody responses. Levitz (2014) used β-glucan as a novel vaccine platform. β-Glucan can be loaded with antigens and immunomodulators such that the “payload” is released following phagocytosis. They have demonstrated robust and long-lasting antigen-specific T cell and antibody responses following immunization of mice with β-glucan “encapsulated” with antibody.
5.3. Applications in cosmetics
β-Glucan has been used in general protective creams, ointments, powders and suspensions for several decades. The attributed activity of β-glucan if that it increases collagen production, and reduces age-lines, crows feet, wrinkles, cellulite, acne, dermatitis, eczema, psoriasis and other skin conditions. It was found that β-glucan is a film-forming moisturizer and a promoter of wound healing. Pillai, Redmond, and Röding (2005) found that the penetration of oat β-glucan in human skin models and to evaluate clinically its efficacy for reducing fine-lines and wrinkles. The results indicated that β-glucan deeply penetrated the skin into the epidermis and dermis. The research supports the use of β-glucan in the care and maintenance of healthy skin and the cosmetic treatment of the signs of aging. One study showed that a low concentration of a specific polysaccharide of the sclero-glucan class is useful in the field of skin and dermatological compositions. The glucan composition indicated advantageous effects, such as moisturization of skin or mucosa, and has an anti-aging and revitalizing effect on the skin (Baschong, Monglat, & Ochs, 2009). Chen (2014) found a mushroom glucan has great moisture retention and can be used in eye drops for alleviating xerophthalmia. Du et al. (2014) also reviewed the skin health promotion effects of natural β-glucan derived from cereals and microorganisms.
Park et al. (2001) obtained a β-1, 6-branched-β-1, 3-glucan from liquid mycelia culture of S. commune. The obtained results demonstrated that this glucan can defer skin aging, impart skin whitening effect and cure skin damage effectively. Ketkeaw, Oungbho, and Wititsuwannakul (2012) found that a β-glucan from Hevea latex increased fibroblast collagen production as compared to control. The results suggested that possible application of the Hevea β-glucan for treatment involving collagen loss in aging skin. Vacharaprechakul, Krisdaphong, and Kanlayavattanakul (2007) formulated a kind of cosmetics containing brewer’s yeast CM glucan. The stability and preference was tested and was clinically evaluated. It was reported that the formulation containing 0.1% of CM glucan was stable with good spread ability and skin soothing effect. Kanlayavattanakul and Lourith (2008) demonstrated the anti-wrinkle efficacy of 0.04% CM glucan in o/w emulsion using testing on 10 volunteers aged over 60 years by twice daily application around eyes and forearm. The results showed that the 0.04% CM glucan emulsion significantly improved skin condition within 28 days as the skin was firmer and the eye wrinkle depth was reduced. In a study by Medeiros et al. (2013), the effects of the β-glucan from the baker’s yeast on wound healing were assessed in human venous ulcers. As a result, β-glucan enhanced ulcer healing and increased epithelial hyperplasia, as well as increased inflammatory cells, angiogenesis and fibroblast proliferation.
5.4. Applications in other health products
Suzuki, Nakamura, Nakayama, and Nishikawa (2005) found a kind of β-1, 3-1, 6-D-glucan which was useful for materials for health foods obtaining from the culture medium containing β-glucan, which a microorganism belonging to Aureobasidium sp. produces extracellularly. Additionally, Mitra (2008) investigated the personal care compositions containing skin and hair care actives such as β-glucans. Such compositions are useful for regulating the condition of mammalian keratinous tissue needing such treatments. Recently, the majority of prebiotics in the markets are derived from non-digestible oligosaccharides. The β-glucan has been reported to be associated with many health-promoting and prebiotic properties. Lam and Cheung (2014) discussed the potential of long chain β-glucans to serve as novel prebiotics based on current knowledge on their sources, preparation, fermentation characteristics, and the plausible mechanisms involved in the utilization. Anusuya and Sathiyabama (2014) demonstrated the preparation of β-glucan (isolated from the cell wall of Pythium aphanidermatum) nanoparticles through the addition of sodium hydroxide to β-glucan solution with constant stirring at 90 °C. Furthermore, the prepared nanoparticles exhibited antifungal activity against P. aphanidermatum. Camelini et al. (2005) investigated the β-glucans from Agaricus brasiliensis in different stages of fruiting body maturity and their use in nutraceutical products. The results showed that mature fruiting bodies of A. brasiliensis should be used for nutraceutical products because they contain these important glucans. Cap-opened, more fragile mature fruiting bodies of A. brasiliensis should be selected over immature ones for the production of nutraceuticals.
5.5. Applications in feed industry
β-Glucans have been produced not only in the food and pharmaceutical industries as anticancer and immuno-modulating agents, but in the aquaculture and livestock industries for enhancing the natural immunity of animals (Jung et al., 2007). Jung et al. (2007) suggested that the β-glucan from Paenibacillus polymyxa JB115 could be used as an animal feed additive for the purpose of enhancing immunity and as a potential antitumor agent for livestock. Moreover, rainbow trout (Oncorhynchus mykiss) were fed pellets containing β-glucan at a dose of 0.5 g/100 g of pellets (0.5%) per day. The results found that β-glucan increase the number of specific antibody secreting cells and specific Ig levels in serum (Siwicki et al., 2004).
6. Conclusions
In general, this review provides the consumer with a higher diversity of glucans; allow farmers an efficient and profitable use of the mushroom biomass. Advancement in the production process and functional properties of β-glucan will greatly enhance our ability to evaluate present and future β-glucan products. Taken together, screening and modification for the most potent and cost-effective β-glucans could then be made efficient. Promising results of clinical experiment in wound healing suggest that further in vivo experiments should be performed. The study also has been an attractive focus of developing β-glucan products that can aid in cancer treatment and has also performed research on the ability of β-glucans to assist in anti-inflammatory activity. Additionally, cereal β-glucan showed a great potential as a thickener or stabilizer in food products. However, further research will focus on its performance in food systems. It is hard to maintain consistency, reproducibility and reliability of different β-glucans due to the variable activities of raw material sources and the structure of β-glucan. Hence, it is necessary to establish the standard protocols for collection of the sources and for the extraction, isolation, purification and preparation of β-glucan. These will be useful for β-glucan applications in food, medicine and cosmetic products. To make better use of β-glucan, food manufacturers and processors must bring attention not only to ensure sufficient concentration of β-glucan in the raw material but also to the processing methods and physicochemical properties of β-glucan, decreasing mechanical and enzymatic breakdown of the β-glucans in end-product and optimizing processing conditions. Additionally, it is important for food scientists to develop alternative polysaccharide sources and the best use of polysaccharide to increase their digestibility and bioavailability.
Acknowledgments
This research was jointly supported by a research grant (R201402) from Beijing Normal University-Hong Kong Baptist University United International College, China, National Natural Science Foundation of China (31470542) and Natural Science Foundation of Hebei Province (C2014407059).
References
- Adachi et al., 2013M. Adachi, W. Kowhakul, H. Masamoto, M. ShigematsuBioactivities of β-glucan and tannin extracted with superheated water by using a macchinetta extractor4th international conference on biology, environment and chemistry, 58, IACSIT Press, Singapore (2013), pp. 71-76
- Ahmad, 2014J. AhmadEffect of particle size and temperature on rheology and creep behavior of barley β-D-glucan concentrate doughCarbohydrate Polymers, 111 (2014), pp. 89-100
- Ahmad et al., 2010A. Ahmad, F.M. Anjum, T. Zahoor, H. Nawaz, Z. AhmedExtraction and characterization of β-D-glucan from oat for industrial utilizationInternational Journal of Biological Macromolecules, 46 (2010), pp. 304-309
- Ahmad et al., 2012A. Ahmad, F.M. Anjum, T. Zahoor, H. Nawaz, S.M. DilshadBeta glucan: a valuable functional ingredient in foodsCritical Reviews in Food Science and Nutrition, 52 (2012), pp. 201-212
- Ahmad et al., 2009A. Ahmad, F.M. Anjum, T. Zahoor, H. Nawaz, A. DinPhysicochemical and functional properties of barley β-glucan as affected by different extraction proceduresInternational Journal of Food Science and Technology, 44 (2009), pp. 181-187
- Ahmad et al., 2015N.H. Ahmad, S. Mustafa, Y.B. Che ManMicrobial polysaccharides and their modification approaches: a reviewInternational Journal of Food Properties, 18 (2015), pp. 332-347
- Anusuya and Sathiyabama, 2014S. Anusuya, M. SathiyabamaPreparation of β-D-glucan nanoparticles and its antifungal activityInternational Journal of Biological Macromolecules, 70 (2014), pp. 440-443
- Bahl et al., 2009Bahl, A. K., Vercellotti, S. V., Vercellotti, J. R., & Klein, E. (2009). Methods of purifying beta-glucans. US patent 7550584 B2.
- Barone Lumaga et al., 2012R. Barone Lumaga, D. Azzali, V. Fogliano, L. Scalfi, P. VitaglioneSugar and dietary fibre composition influence, by different hormonal response, the satiating capacity of a fruit-based and a β-glucan-enriched beverageFood and Function, 3 (2012), pp. 67-75
- Barsanti et al., 2011L. Barsanti, V. Passarelli, V. Evangelista, A.M. Frassanito, P. GualtieriChemistry, physico-chemistry and applications linked to biological activities of β-glucansNatural Product Reports, 28 (2011), pp. 457-466
- Baschong et al., 2009Baschong, W., Monglat, S., & Ochs, D. (2009). Glucan compositions. US patent 0156563 A1.
- Beer et al., 1996M.U. Beer, E. Arrigoni, R. AmadoExtraction of oat gum from oat bran: effects of process on yield, molecular weight distribution, viscosity and (1→3)(1→4)-β-D-glucan content of the gumCereal Chemistry, 73 (1996), pp. 58-62
- Belcarz et al., 2013A. Belcarz, G. Ginalska, T. Pycka, A. Zima, A. Ślósarczyk, I. Polkowska, et al.Application of β-1,3-glucan in production of ceramics-based elastic composite for bone repairCentral European Journal of Biology, 8 (2013), pp. 534-548
- Berdal et al., 2007M. Berdal, H.I. Appelbom, J.H. Eikrem, A. Lund, S. Zykova, L.T. Busund, et al.Aminated beta-1,3-D-glucan improves wound healing in diabetic db/db miceWound Repair and Regeneration, 15 (2007), pp. 825-832
- Bhanja et al., 2014S.K. Bhanja, D. Rout, P. Patra, I.K. Sen, C.K. Nandan, S.S. IslamWater-insoluble glucans from the edible fungus Ramaria botrytisBioactive Carbohydrate and Dietary Fibre, 3 (2014), pp. 52-58
- Bhatty, 1992R.S. BhattyBeta-glucan content and viscosities of barleys and their roller-milled flour and bran productsCereal Chemistry, 69 (1992), pp. 469-471
- Bhatty, 1996Bhatty, R. S. (1996). Methods for extracting cereal β-glucans. US patent 5518710.
- Bohn and BeMiller, 1995J.A. Bohn, J.N. BeMiller(1→3)-beta-D-glucans as biological response modifiers: a review of structure-functional activity relationshipsCarbohydrate Polymers, 28 (1995), pp. 3-14
- Brennan and Cleary, 2005C.S. Brennan, L.J. ClearyThe potential use of (1→3, 1→4)-β-D-glucans as functional food ingredientsJournal of Cereal Science, 42 (2005), pp. 1-13
- Brennan et al., 2013M.A. Brennan, E. Derbyshire, B.K. Tiwari, C.S. BrennanIntegration of β-glucan fibre rich fractions from barley and mushrooms to form healthy extruded snacksPlant Foods for Human Nutrition, 68 (2013), pp. 78-82
- Brummer et al., 2014Y. Brummer, C. Defelice, Y. Wu, M. Kwong, P.J. Wood, S.M. ToshTextural and rheological properties of oat beta-glucan gels with varying molecular weight compositionJournal of Agricultural and Food Chemistry, 62 (2014), pp. 3160-3167
- Cahill et al., 2003Cahill, A. P., Fenske, D. J., Freeland, M., & Hartwig, G. W. (2003). Beta-glucan process, additive and food product. US patent 6749885 B2.
- Camelini et al., 2005C.M. Camelini, M. Maraskin, M.M. De Mendonca, C. Zucco, A.G. Ferreira, L.A. TavaresStructural characterization of beta-glucans of Agaricus brasiliensis in different stages of fruiting body maturity and their use in nutraceutical productsBiotechnology Letters, 27 (2005), pp. 1295-1299
- Chakraborty et al., 2004I. Chakraborty, S. Mondal, M. Pramanik, D. Rout, S.S. IslamStructural investigation of a water-soluble glucan from an edible mushroom, Astraeus hygrometricusCarbohydrate Research, 9 (2004), pp. 2249-2254
- Chakraborty et al., 2006I. Chakraborty, S. Mondal, D. Rout, S.S. IslamA water-insoluble (1→3)-beta-D-glucan from the alkaline extract of an edible mushroom Termitomyces eurhizusCarbohydrate Research, 341 (2006), pp. 2990-2993
- Chauveau et al., 1996C. Chauveau, P. Talaga, J.M. Wieruszeski, G. Strecker, L. ChavantA water-soluble beta-D-glucan from Boletus erythropusPhytochemistry, 43 (1996), pp. 413-415
- Chen, 2014Chen, S. N. (2014). Composite glucan and method for preparing the same. US patent 20140031542 A1.
- Cheung, 2013Cheung, N. K. (2013). Therapy-enhancing glucan. US patent 20130230535 A1.
- Chihara, 2001G. ChiharaInternational journal of oriental medicineOriental Healing Arts Institute of U.S.A Press (2001), pp. 261-266
- Cox, 2013Cox, D. J. (2013). Particulate-soluble glucan preparation. US patent 20130237497 A1.
- Crognale et al., 2007S. Crognale, M. Bruno, M. Fidaleo, M. Moresi, M. PetruccioliProduction of β-glucan and related glucan-hydrolases by Botryosphaeria rhodinaJournal of Applied Microbiology, 102 (2007), pp. 860-871
- Cui et al., 2000W. Cui, P.J. Wood, B. Blackwell, J. NikiforukPhysicochemical properties and structural characterization by two-dimensional NMR spectroscopy of wheat β-D-glucan-comparison with other cereal β-D-glucansCarbohydrate Polymers, 41 (2000), pp. 249-258
- Daou and Zhang, 2012C. Daou, H. ZhangOat beta-glucan: its role in health promotion and prevention of diseasesComprehensive Reviews in Food Science and Food Safety, 11 (2012), pp. 355-365
- Dawkins and Nnanna, 1993N.L. Dawkins, I.A. NnannaOat gum and β-glucan extraction from oat bran and rolled oats: temperature and pH effectsJournal of Food Science, 58 (1993), pp. 562-566
- Delatte et al., 2001S.J. Delatte, J. Evans, A. Hebra, W. AdamsonEffectiveness of beta-glucan collagen for treatment of partial-thickness burns in childrenJournal of Pediatric Surgery, 36 (2001), pp. 113-118
- Ding et al., 2013J.Z. Ding, Y.F. Wang, S.B. Xiong, S.M. Zhao, Q.L. HuangOptimised methodology for carboxymethylation of (1→3)-β-D-glucan from yeast (Saccharomyces cerevisiae) and promotion of mechanical activationInternational Journal of Food Science and Technology, 48 (2013), pp. 253-259
- Donzis, 1996Donzis, B. A. (1996). Substantially purified beta (1,3) finely ground yeast cell wall glucan composition with dermatological and nutritional uses. US patent 5576015.
- Du et al., 2014B. Du, Z.X. Bian, B.J. XuSkin health promotion effects of natural beta-glucan derived from cereals and microorganisms: a reviewPhytotherapy Research, 28 (2014), pp. 159-166
- Du et al., 2015B. Du, C.Y. Lin, Z.X. Bian, B.J. XuAn insight into anti-inflammatory effects of fungal beta-glucanTrends in Food Science & Technology, 41 (2015), pp. 49-59
- Du and Xu, 2014B. Du, B.J. XuOxygen radical absorbance capacity (ORAC) and ferric reducing antioxidant power (FRAP) of β-glucans from different sources with various molecular weightBioactive Carbohydrate and Dietary Fibre, 3 (2014), pp. 11-16
- Du et al., 2014aB. Du, F.M. Zhu, B.J. Xuβ-Glucan extraction from bran of hull-less barley by accelerated solvent extraction combined with response surface methodologyJournal of Cereal Science, 59 (2014), pp. 95-100
- Du et al., 2014bB. Du, F.M. Zhu, B.J. XuPhysicochemical and antioxidant properties of dietary fibers from Qingke (hull-less barley) flour as affected by ultrafine grindingBioactive Carbohydrate and Dietary Fibre, 4 (2014), pp. 170-175
- Garcia-Ochoa et al., 2000F. Garcia-Ochoa, E. Gomez Castro, V.E. SantosOxygen transfer and uptake rates during Xanthan gum productionEnzyme and Microbial Technology, 27 (2000), pp. 680-690
- Genç et al., 2001H. Genç, M. Ozdemir, A. DemirbasAnalysis of mixed linked (1→3)(1→4)-β-D-glucans in cereal grains from TurkeyFood Chemistry, 73 (2001), pp. 221-224
- Georing and Eslick, 1988Georing, K. J., & Eslick, R. F. (1988). Production of beta-glucan, bran, protein, oil and maltose syrup from waxy barley. US patent 4804545.
- Hozova et al., 2007B. Hozova, L. Kuniak, P. Morvcikova, A. GajdosovaDetermination of water insoluble β-D-glucan in the whole grain cereals and pseudocerealsCzech Journal of Food Sciences, 25 (2007), pp. 316-324
- Immerstrand et al., 2009T. Immerstrand, B. Bergenstahl, C. Tragardh, M. Nyman, S. Cui, R. OsteExtraction of β-glucan from oat bran on laboratory scaleCereal Chemistry, 86 (2009), pp. 601-608
- Irakli et al., 2004M. Irakli, C.G. Biliaderis, M.S. Izydorczyk, I.N. PapadoyannisIsolation, structural features and rheological properties of water-extractable β-glucans from different Greek barley cultivarsJournal of the Science of Food and Agriculture, 84 (2004), pp. 1170-1178
- Jamas et al., 1997Jamas, S., Easson, D. D., & Ostroff, G. R. (1997). Glucan preparation. US patent 5622939.
- Jamas et al., 1998Jamas, S., Easson, D. D., & Ostroff, G. R. (1998). Method for producing soluble glucans. US patent 5633369.
- Jung et al., 2007H.K. Jung, J.H. Hong, S.C. Park, B.K. Park, D.H. Nam, S.D. KimProduction and physicochemical characterization of β-glucan produced by Paenibacillus polymyxa JB115Biotechnology and Bioprocess Engineering, 12 (2007), pp. 713-719
- Kalyanasundaram et al., 2012G.T. Kalyanasundaram, M. Doble, S.N. GummadiProduction and downstream processing of (1→3)-β-D-glucan from mutant strain of Agrobacterium sp. ATCC 31750AMB Express, 2 (2012), p. 31
- Kanlayavattanakul and Lourith, 2008M. Kanlayavattanakul, N. LourithCarboxymethylglucan in cosmeticsThai Pharmaceutical and Health Science Journal, 3 (2008), pp. 378-382
- Kao et al., 2012P.F. Kao, S.H. Wang, W.T. Hung, Y.H. Liao, C.M. Lin, W.B. YangStructural characterization and antioxidative activity of low-molecular-weights beta-1,3-glucan from the residue of extracted Ganoderma lucidum fruiting bodiesBioMed Research International, 2012 (2012), pp. 1-8
- Kawagishi et al., 1990H. Kawagishi, T. Kanao, R. Inagaki, T. MizunoFormolysis of a potent antitumor (1→6)-β-D-glucan-protein complex from Agaricus blazei fruiting bodies and antitumor activity of the resulting productsCarbohydrate Polymers, 12 (1990), pp. 393-403
- Kelly, 2006Kelly, G. E. (2006). Novel therapeutic uses of glucan. US patent 0293278 A1.
- Ketkeaw et al., 2012R. Ketkeaw, K. Oungbho, R. WititsuwannakulThe β-glucan from Hevea brasiliensis latex and its possible application in anti-aging cosmeceuticals38th Congress on science and technology of Thailand (2012)
- Kim et al., 2013S.R. Kim, H.W. Kang, H.S. RoGeneration and evaluation of high β-glucan producing mutant strains of Sparassis crispaMycobiology, 41 (2013), pp. 159-163
- Kim et al., 2005Y.W. Kim, K.H. Kim, H.J. Choi, D.S. LeeAnti-diabetic activity of β-glucans and their enzymatically hydrolyzed oligosaccharides from Agaricus blazeiBiotechnology Letters, 27 (2005), pp. 483-487
- Kim et al., 2011J. Kim, S.M. Lee, I.Y. Bae, H.G. Park, H. Gyu Lee, S. Lee(1-3)(1-6)-β-glucan-enriched materials from Lentinus edodes mushroom as a high-fibre and low-calorie flour substitute for baked foodsJournal of the Science of Food and Agriculture, 91 (2011), pp. 1915-1919
- Kim et al., 2012H.L. Kim, J.H. Lee, M.H. Lee, B.J. Kwon, J.C. ParkEvaluation of electrospun (1,3)-(1,6)-β-D-glucans/biodegradable polymer as artificial skin for full-thickness wound healingTissue Engineering Part A, 18 (2012), pp. 2315-2322
- Kim et al., 2008Kim, M. S., Park, Y. D., & Lee, S. R. (2008). Preparation method of beta-glucan from Schizophyllum commune and composition for experimental application comprising the same. US patent 0160043 A1.
- Kim et al., 2009Kim, M. S., Park, Y. D., & Lee, S. R. (2009). Method of using beta-glucan from Schizophyllum commune. US patent 0023681 A1.
- Kishida et al., 1992E. Kishida, Y. Sone, A. MisakiEffects of branch distribution and chemical modifications of antitumor (1→3)-β-D-glucansCarbohydrate Polymers, 17 (1992), pp. 89-95
- Kittisuban et al., 2014P. Kittisuban, P. Ritthiruangdej, M. SuphantharikaOptimization of hydroxypropylmethylcellulose, yeast β-glucan, and whey protein levels based on physical properties of gluten-free rice bread using response surface methodologyLWT – Food Science and Technology, 57 (2014), pp. 738-748
- Kofuji et al., 2010K. Kofuji, Y. Huang, K. Tsubaki, F. Kokido, K. Nishikawa, T. Isobe, et al.Preparation and evaluation of a novel wound dressing sheet comprised of β-glucan–chitosan complexReactive and Functional Polymers, 70 (2010), pp. 784-789
- Kogan, 2000G. Kogan(1–3, 1-6)-β-D-glucans of yeasts and fungi and their biological activityAtta-ur Rahman (Ed.), Studies in natural products chemistry, Vol. 23, Elsevier Science B.V., Amsterdam (2000), pp. 107-151
- Kogan et al., 2005G. Kogan, A. Staško, K. Bauerová, M. Polovka, L. Šoltés, V. Brezová, et al.Antioxidant properties of yeast (1→3)-β-D-glucan studied by electron paramagnetic resonance spectroscopy and its activity in the adjuvant arthritisCarbohydrate Polymers, 61 (2005), pp. 18-28
- Kumari et al., 2008M. Kumari, S.A. Survase, R.S. SinghalProduction of schizophyllan using Schizophyllum commune NRCMBioresource Technology, 99 (2008), pp. 1036-1043
- Lam and Cheung, 2014K.L. Lam, C.K. CheungNon-digestible long chain beta-glucans as novel prebioticsBioactive Carbohydrate and Dietary Fibre, 2 (2014), pp. 45-64
- Lazaridou and Biliaderis, 2007A. Lazaridou, C.G. BiliaderisMolecular aspects of cereal β-glucan functionality: physical properties, technological applications and physiological effectsJournal of Cereal Science, 46 (2007), pp. 101-118
- Lazaridou et al., 2003A. Lazaridou, C.G. Biliaderis, M.S. IzydorczykMolecular size effects on rheological properties of oat β-glucans in solution and gelsFood Hydrocolloids, 17 (2003), pp. 693-712
- Lazaridou et al., 2014A. Lazaridou, A. Marinopoulou, N.P. Matsoukas, C.G. BiliaderisImpact of flour particle size and autoclaving on β-glucan physicochemical properties and starch digestibility of barley rusks as assessed by in vitro assaysBioactive Carbohydrate and Dietary Fibre, 4 (2014), pp. 58-73
- Lee et al., 2001J.N. Lee, D.Y. Lee, I.H. Ji, G.E. Kim, H.N. Kim, J.S. Sohn, et al.Purification of soluble β-glucan with immune-enhancing activity from the cell wall of yeastBioscience Biotechnology, and Biochemistry, 65 (2001), pp. 837-841
- Leung et al., 2006M.Y.K. Leung, C. Liu, J.C.M. Koon, K.P. FungPolysaccharide biological response modifiersImmunology Letters, 105 (2006), pp. 101-114
- Levitz, 2014S. Levitzβ-Glucan particles as a vaccine platform with intrinsic adjuvanticity (469.3)FASEB Journal, 28 (2014), pp. 469-473
- Liu, 2010Y.J. LiuBeta-glucan effects on pasting properties and potential health benefits of flours from different oat linesIowa State University (2010)Graduate Theses and Dissertations. Paper 11303
- Liu et al., 2014Y. Liu, J. Zhang, Q. Tang, Y. Yang, Q. Guo, Q. Wang, et al.Physicochemical characterization of a high molecular weight bioactive β-D-glucan from the fruiting bodies of Ganoderma lucidumCarbohydrate Polymers, 101 (2014), pp. 968-974
- Medeiros et al., 2013S.D.V. Medeiros, S.L. Cordeiro, J.E. Cavalcanti, K.M. Melchuna, A.M. Lima, I.A. Filho, et al.Effects of purified Saccharomyces cerevisiae (1→3)-β-glucan on Vevnous ulcer healingInternational Journal of Molecular Sciences, 13 (2013), pp. 8142-8158
- Min et al., 2012J.H. Min, Y.H. Kim, J.H. Kim, S.Y. Choi, J.S. Lee, H.K. KimComparison of microbial diversity of Korean commercial Makgeolli showing high β-glucan content and high antihypertensive activity, respectivelyMycobiology, 40 (2012), pp. 138-141
- Misaki et al., 1988Misaki, A., Sone, Y., Yoshida, M., & Takeuchi, K. (1988). Beta-glucan. US patent 4769363.
- Mithöfer et al., 1996A. Mithöfer, F. Lottspeich, J. EbelOne-step purification of the β-glucan elicitor-binding protein from soybean (Glycine max L.) roots and characterization of an anti-peptide antiserumFEBS Letter, 381 (1996), pp. 203-207
- Mitra, 2008Mitra, S. (2008). Personal care compositions comprising alpha-glucans and/or beta-glucans. US patent 0095731 A1.
- Mohanty et al., 2015J. Mohanty, P.K. Sahoo, B.R. Pillai, S. Mohanty, S.K. Garnayak, S. KumarPurification and characterization of a β-glucan binding protein from the haemolymph of freshwater prawn Macrobrachium rosenbergiiAquaculture Research, 46 (2015), pp. 95-104
- Morgan, 2002Morgan, K. R. (2002). β-Glucan products and extraction process from cereals. US patent 0192770 A1.
- Morgan, 2006Morgan, K. R. (2006). Process for extraction of β-glucan from cereals and products obtained therefrom. US patent 7138519 B2.
- Ookushi et al., 2006Y. Ookushi, M. Sakamoto, J. AzumaOptimization of microwave-assisted extraction of polysaccharides from the fruiting body of mushroomsJournal of Applied Glycoscience, 53 (2006), pp. 267-272
- Park et al., 2009S.Y. Park, I.Y. Bae, S. Lee, H.G. LeePhysicochemical and hypocholesterolemic characterization of oxidized oat β-glucanJournal of Agricultural and Food Chemistry, 57 (2009), pp. 439-443
- Park et al., 2014H. Park, K.H. Ka, S.R. RyuEnhancement of β-glucan content in the cultivation of cauliflower mushroom (Sparassis latifolia) by elicitationMycobiology, 42 (2014), pp. 41-45
- Park et al., 2001Park, K. M., Park, B. H., So, S., Kim, M. S., Kim, J. S., Kim, Y. T., et al. (2001). Composition for external application containing a beta-1,6-branched-beta-1,3-glucan. US patent 0029253 A1.
- Pillai et al., 2005R. Pillai, M. Redmond, J. RödingAnti-wrinkle therapy: significant new findings in the non-invasive cosmetic treatment of skin wrinkles with beta-glucanInternational Journal of Cosmetic Science, 27 (2005), p. 292
- Potter et al., 2003Potter, R. C., Fisher, P. A., Hash, K. R., & Neidt, J. D. (2003). Method for concentrating β-glucan. US patent 6323338 B1.
- Redmond and Fielder, 2006Redmond, M. J., & Fielder, D. A. (2006). Extraction and purification method for cereal beta-glucan. US patent 012149 A1.
- Rieder and Samuelsen, 2012A. Rieder, A.B. SamuelsenDo cereal mixed-linked β-glucans possess immune-modulating activities?Molecular Nutrition & Food Research, 56 (2012), pp. 536-547
- Rinaldi et al., 2015L. Rinaldi, L.E. Rioux, M. Britten, S.L. TurgeonIn vitro bioaccessibility of peptides and amino acids from yogurt made with starch, pectin, or β-glucanInternational Dairy Journal, 46 (2015), pp. 39-45
- Routray and Orsat, 2012W. Routray, V. OrsatMicrowave-assisted extraction of flavonoids: a reviewFood and Bioprocess Technology, 5 (2012), pp. 409-424
- Sakurai et al., 2001K. Sakurai, M. Mizu, S. ShinkaiPolysaccharide-polynucleotide complexes. 2. Complementary polynucleotide mimic behavior of the natural polysaccharide schizophyllan in the macromolecular complex with single-stranded RNA and DNABiomacromolecules, 2 (2001), pp. 641-650
- Samuelsen et al., 2014A.B. Samuelsen, J. Schrezenmeir, S.H. KnutsenEffects of orally administered yeast-derived beta-glucans: a reviewMolecular Nutrition & Food Research, 58 (2014), pp. 183-193
- Sarteshnizia et al., 2015R.A. Sarteshnizia, H. Hosseinia, D. Bondarianzadeha, F.J. Colmenerob, R. KhaksaraOptimization of prebiotic sausage formulation: effect of using β-glucan and resistant starch by D-optimal mixture design approachLWT – Food Science and Technology, 62 (2015), pp. 704-710
- Sato et al., 2012H. Sato, Y. Kobayashi, A. Hattori, T. Suzuki, M. Shigekawa, T. JippoInhibitory effects of water-soluble low-molecular-weight β-(1,3-1,and 6) D-glucan isolated from Aureobasidium pullulans 1A1 strain black yeast on mast cell degranulation and passive cutaneous anaphylaxisBioscience Biotechnology and Biochemistry, 76 (2012), pp. 84-88
- Saulnier et al., 1994L. Saulnier, S. Gévaudan, J.F. ThibaultExtraction and partial characterization of β-glucan from the endosperms of two barley cultivarsJournal of Cereal Science, 19 (1994), pp. 171-178
- Sen et al., 2013I.K. Sen, P.K. Maji, B. Behera, T.K. Maiti, P. Mallick, S.R. Sikdar, et al.Glucan of a somatic hybrid mushroom, pfls1h: structural characterization and study of immunological activitiesInternational Journal of Biological Macromolecules, 53 (2013), pp. 127-132
- Sharafbafi et al., 2014H. Sharafbafi, S.M. Tosh, M. Alexander, M. CorredigPhase behaviour, rheological properties, and microstructure of oat β-glucan-milk mixturesFood Hydrocolloids, 41 (2014), pp. 274-280
- Sharma and Gujral, 2013P. Sharma, H.S. GujralExtrusion of hulled barley affecting β-glucan and properties of extrudatesFood and Bioprocess Technology, 6 (2013), pp. 1374-1389
- Sibakov et al., 2012J. Sibakov, O. Myllymaki, U. Holopainen, A. Kaukovirta-norja, V. Hietaniemi, J.M. Pihlava, et al.Minireview: β-Glucan extraction methods from oatsAgro Food Industry Hi-Tech, 23 (2012), pp. 10-12
- Siwicki et al., 2004A.K. Siwicki, K. Kazuñ, E. Glbski, E. Terech-Majewska, P. Baranowski, S. TrapkowskaThe effect of beta-1.3/1.6-glucan in diets on the effectiveness of anti-yersinia ruckeri vaccine-an experimental study in rainbow trout (Oncorhynchus mykiss)Polish Journal of Food and Nutrition Sciences, 13 (2004), pp. 59-61
- Skendi et al., 2003A. Skendi, C.G. Biliaderis, A. Lazaridou, M.S. IzydorczykStructure and rheological properties of water soluble β-glucans from oat cultivars of Avena sativa and Avena byzantinaJournal of Cereal Science, 38 (2003), pp. 15-31
- Smiderle et al., 2006F.R. Smiderle, E.R. Carbonero, C.G. Mellinger, G.L. Sassaki, P.A. Gorin, M. IacominiStructural characterization of a polysaccharide and a beta-glucan isolated from the edible mushroom Flammulina velutipesPhytochemistry, 67 (2006), pp. 2189-2196
- Soltanian et al., 2009S. Soltanian, E. Stuyven, E. Cox, P. Sorgeloos, P. BossierBeta-glucans as immunostimulant in vertebrates and invertebratesCritical Reviews in Microbiology, 35 (2009), pp. 109-138
- Stack et al., 2010H.M. Stack, N. Kearney, C. Stanton, G.F. Fitzgerald, R.P. RossAssociation of beta-glucan endogenous production with increased stress tolerance of intestinal LactobacilliApplied and Environmental Microbiology, 76 (2010), pp. 500-507
- Suphantharika et al., 2003M. Suphantharika, P. Khunrae, P. Thanardkit, C. VerduynPreparation of spent brewer’s yeast β-glucans with a potential application as an immunostimulant for black tiger shrimp, Penaeus monodonBioresource Technology, 88 (2003), pp. 55-60
- Suzuki et al., 2005Suzuki, T., Nakamura, S., Nakayama, S., & Nishikawa, K. (2005). Beta-1,3–1,6-D-glucan and its use. US patent 0271613 A1.
- Synytsya and Novák, 2013A. Synytsya, M. NovákStructural diversity of fungal glucansCarbohydrate Polymers, 92 (2013), pp. 792-809
- Temelli, 1997F. TemelliExtraction and functional properties of barley β-glucan as affected by temperature and pHJournal of Food Science, 62 (1997), pp. 1192-1201
- Thammakiti et al., 2004S. Thammakiti, M. Suphantharika, T. Phaesuwan, C. VerduynPreparation of spent brewer’s yeast β-glucans for potential applications in the food industryInternal Journal of Food Science and Technology, 39 (2004), pp. 21-29
- Tian et al., 2012Y.T. Tian, H.L. Zeng, Z.B. Xu, B.D. Zheng, Y.X. Lin, C.J. Gand, et al.Ultrasonic-assisted extraction and antioxidant activity of polysaccharides recovered from white button mushroom (Agaricus bisporus)Carbohydrate Polymers, 88 (2012), pp. 522-529
- Toklu et al., 2006H.Z. Toklu, G. Sener, N. Jahovic, B. Uslu, S. Arbak, B.C. Yeğenbeta-glucan protects against burn-induced oxidative organ damage in ratsInternational Immunopharmacology, 6 (2006), pp. 156-169
- Tungland and Meyer, 2002B.C. Tungland, D. MeyerNon-digestible oligo- and polysaccharides (dietary fiber): their physiology and role in human health and foodComprehensive Reviews in Food Science and Food Safety, 1 (2002), pp. 90-109
- Vacharaprechakul et al., 2007V. Vacharaprechakul, P. Krisdaphong, M. KanlayavattanakulThe development and clinical evaluation of carboxymethyl glucanM. Sc. (Cosmetic Science) Independence StudyMae Fah Luang University, Chiang Rai (2007)
- Van Lengerich et al., 2004Van Lengerich, B. H., Gruess, O., & Meuser, F. P. (2004). Beta-glucan compositions and process therefore. US patent 6835558 B2.
- Vasanthan and Temelli, 2001Vasanthan, T., & Temelli, F. (2001). Grain fractionation methods and products. European patent 1366081 B1.
- Vasanthan and Temelli, 2008T. Vasanthan, F. TemelliGrain fractionation technologies for cereal beta-glucan concentrationFood Research International, 41 (2008), pp. 876-881
- Venkatachalam et al., 2013G. Venkatachalam, S. Narayanan, M. DobleApplications of cyclic β-glucans, in name (1st), cyclic β-glucans from microorganisms: Production, properties and applicationsSpringer International Publishing (2013)
- Vetvicka et al., 2007V. Vetvicka, B. Dvorak, J. Vetvickova, J. Richter, J. Krizan, P. Sima, et al.Orally administered marine (1→3)-beta-D-glucan phycarine stimulates both humoral and cellular immunityInternational Journal of Biological Macromolecules, 40 (2007), pp. 291-298
- Wang et al., 2014Q. Wang, S. Chen, L. Han, M.T. Lian, Z.L. Wen, T. Jiayinaguli, et al.Antioxidant activity of carboxymethyl (1→3)-β-D-glucan (from the sclerotium of Poria cocos) sulfate (in vitro)International Journal of Biological Macromolecules, 69 (2014), pp. 229-235
- Watanabe et al., 2013T. Watanabe, R. Shimada, A. Matsuyama, M. Yuasa, H. Sawamura, E. Yoshida, et al.Antitumor activity of the β-glucan paramylon from Euglena against preneoplastic colonic aberrant crypt foci in miceFood and Function, 4 (2013), pp. 1685-1690
- Westerlund et al., 1993E. Westerlund, R. Andersson, P. AmanIsolation and chemical characterization of water-soluble mixed-linked β-glucans and arabinoxylans in oat milling fractionsCarbohydrate Polymers, 20 (1993), pp. 115-123
- Williams et al., 1990Williams, D. L., Browder, I. W., & DiLuzio, N. R. (1990). Soluble phosphorylated glucan: Methods and compositions for wound healing. US patent 4975421.
- Williams et al., 1989Williams, D. L., Browder, I. W., DiLuzio, N. R., & DiLuzio, N. M. (1989). Soluble phosphorylated glucan: Methods and compositions for treatment neoplastic diseases. US patent 4818752.
- Williams et al., 1992D.L. Williams, H.A. Pretus, R.B. McNamee, E.L. Jones, H.E. Ensley, I.W. BrowderDevelopment of a water-soluble, sulfated (1→3)-β-D-glucan biological response modifier derived from Saccharomyces cerevisiaeCarbohydrate Research, 235 (1992), pp. 247-257
- Wood et al., 1994P.J. Wood, J.T. Braaten, F.W. Scott, K.D. Riedel, M.S. Wolynetz, M.W. CollinsEffect of dose and modification of viscous properties of oat gum on plasma glucose and insulin following an oral glucose loadBritish Journal of Nutrition, 72 (1994), pp. 731-743
- Wood et al., 1978P.J. Wood, I.J. Siddiqui, D. PatonExtracting of high-viscosity gums from oatsCereal Chemistry, 55 (1978), pp. 1038-1049
- Wood et al., 1991P.J. Wood, J. Weisz, B.A. BlackwellMolecular characterization of cereal β-glucans. Structural analysis of oat β-glucan and rapid structural evaluation of β-glucans from different sources by high-performance liquid chromatography of oligosaccharides released by lichenaseCereal Chemistry, 68 (1991), pp. 31-39
- Yan et al., 2014J.K. Yan, W.Q. Wang, J.Y. WuRecent advances in Cordyceps sinensis polysaccharides: mycelial fermentation, isolation, structure, and bioactivities: a reviewJournal of Functional Foods, 6 (2014), pp. 33-47
- Yao et al., 2007N. Yao, J.L. Jannink, P.J. WhiteMolecular weight distribution of (1→3)(1→4)-β-glucan affects pasting properties of flour from oat lines with high and typical amounts of β-glucanCereal Chemistry, 84 (2007), pp. 471-479
- Yoshida et al., 2014T. Yoshida, Y. Honda, T. Tsujimoto, H. Uyama, J. AzumaSelective isolation of β-glucan from corn pericarp hemicelluloses by affinity chromatography on cellulose columnCarbohydrate Polymers, 111 (2014), pp. 538-542
- Zeković et al., 2005D.B. Zeković, S. Kwiatkowski, M.M. Vrvić, D. Jakovljević, C.A. MoranNatural and modified (1→3)-beta-D-glucans in health promotion and disease alleviationCritical Reviews in Biotechnology, 25 (2005), pp. 205-230
- Zhu et al., 2015F.M. Zhu, B. Du, Z.X. Bian, B.J. XuBeta-glucans from edible and medicinal mushrooms: characteristics, physicochemical and biological activitiesJournal of Food Composition and Analysis, 41 (2015), pp. 165-173
- Zlatkovic et al., 2003D. Zlatkovic, D. Jakovljevic, D. Zekovic, M.V. MiroslavA glucan from active dry baker’s yeast (Saccharomyces cerevisiae): a chemical and enzymatic investigation of the structureJournal of the Serbian Chemical Society, 68 (2003), pp. 805-809