β-(1 → 3,1 → 6)-d-glucans produced by Diaporthe sp. endophytes: Purification, chemical characterization and antiproliferative activity against MCF-7 and HepG2-C3A cells
Highlights
- •β-glucans (EPS-P) were purified from the fermentation broth of two Diaporthe endophytes.
- •β-glucans consisted of a main chain of glucopyranosyl (1 → 3) linkages substituted at O-6 by glucosyl residues.
- •EPS-PD2 inhibited up to ∼75% of the proliferation of MCF-7 cells.
- •Both glucans inhibited up to ∼84% of the proliferation of HepG2-C3A cells.
Abstract
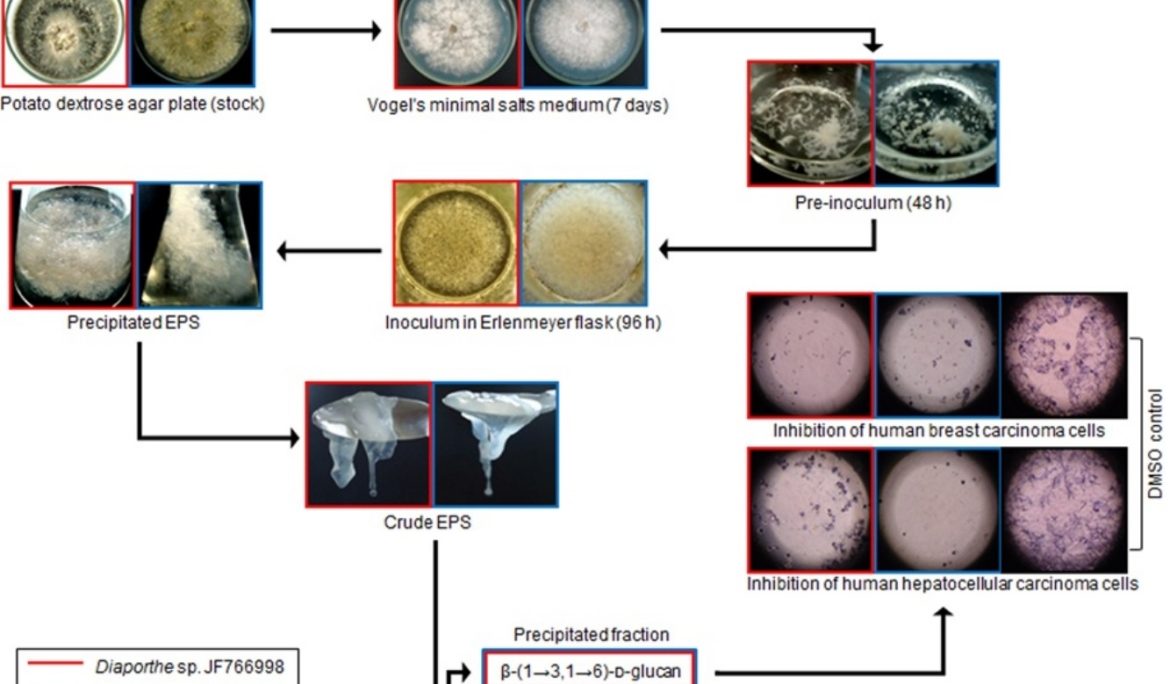
This study reports the characterization and antiproliferative activity of exopolysaccharides (EPS) produced by submerged cultures of the endophytes Diaporthe sp. JF766998 and Diaporthe sp. JF767007 isolated from the medicinal plant Piper hispidum Sw. Both strains secreted a crude EPS that, upon size exclusion chromatography, showed to contain a heteropolysaccharide (galactose, glucose and mannose) and a high-molecular weight glucan. Data from methylation analysis, FTIR and NMR spectroscopy (1H, COSY, TOCSY and HSQC-DEPT) indicated that the purified glucan consisted of a main chain of glucopyranosyl β-(1 → 3) linkages substituted at O-6 by glucosyl residues. According to MTT assay, some treatments of both β-glucans have antiproliferative activity against human breast carcinoma (MCF-7) and hepatocellular carcinoma (HepG2-C3A) cells after 24 and 48 h of treatment, exhibiting a degree of inhibition ratio that reached the highest values at 400 μg/mL: 58.0% (24 h) and 74.6% (48 h) for MCF-7 cells, and 61.0% (24 h) and 83.3% (48 h) for HepG2-C3A cells. These results represent the first reports on the characterization and antiproliferative effect of β-glucans from Diaporthe species and also expand the knowledge about bioactive polysaccharides from endophytic sources.
Graphical abstract

Introduction
The World Health Organization ranked the hepatocellular carcinoma as the second most common cancer-related cause of death worldwide in 2012. On the other hand, breast carcinoma represented the fifth cause of death from cancer overall, being the first and second causes of cancer- death for women in less developed and more developed countries, respectively [1]. It is well known that the use of synthetic drugs as chemotherapeutic agents implies some limitations as the relatively severe side-effects in patients; thus, efforts to find new antitumor drugs with fewer undesirable effects are of great importance for human health.
In this context, fungal glucans are relevant bioactive molecules for their antimicrobial [2], anticancer [3], [4], and glucose-lowering [5] activities. In particular, β-glucans are known to interact with several receptors of immune cells triggering innate and adaptative responses, and are considered as potent modifiers of the immune response [6], [7]. Structurally, fungal β-(1,3)-glucans are made up of a linear backbone of β-(1,3)-glucopyranose randomly branched, generally at O-6 positions, by side chains of variable sizes. Factors like branching degree, molecular mass and tertiary structure affect the bioactive properties of β-glucans and, for example, polymers of high molecular weight seem to exert better antitumor action than smaller ones [8]. These polysaccharides are components of fungal cell walls but some of them, as scleroglucan or schizophyllan, are produced as extracellular polymers, and are of especial interest for being easily recovered from the culture broths.
The medicinal plant Piper hispidum Sw. (called “cordoncillo” in Mexico and “falso-jaborandi” in Brazil) harbors a diversity of endophytes [9], which include isolates that secrete compounds with antimicrobial and enzymatic activity [10], [11]. In a previous paper we identified two of these strains as two different Diaporthe sp. isolates. Both are exopolysaccharides (EPS) producers and one of them secretes a glucose-rich exopolysaccharide (EPS) when incubated for 96 h in submerged cultures [13]. This study reports the production and characterization of β-glucans from these endophytic strains and the results from evaluation of their antiproliferative activity.
Section snippets
Reagents and culture media
Potato dextrose agar medium (PDA) was purchased from HiMedia Labs. (Mumbai, MH, India). Dulbecco’s modified Eagle’s medium (DMEM) and fetal bovine serum (FBS) were purchased from Gibco-Invitrogen Co. (Carlsbad, CA, USA). Analytical standards, dimethyl sulfoxide (DMSO), dimethyl sulfoxide-d6 (DMSO-d6), trifluoroacetic acid (TFA), methyl methanesulphonate (MMS) and 3-(4,5-dimethylthiazol-2-il)-2,5-diphenyl tetrazolium bromide (MTT) were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). All
Production, purification and characteristics of EPS
The crude material secreted by Diaporthe sp. JF766998 (EPS-CD1) and Diaporthe sp. JF767007 (EPS-CD2) contained ≥90% carbohydrate (Table 1). The yield of EPS-CD1 (0.08 g/L) was remarkably higher than that of EPS-CD2 (0.04 g/L), corroborating the marked differences obtained in our previous study [13]. As reported before, the synthesis of fungal polysaccharides is a strain-dependent process [23], what could explain the differences in EPS secreted by closely related species cultured under the same
Conclusions
The current study is the first report on the production, chemical characterization and evaluation of antiproliferative properties of β-glucans from Diaporthe genus. Two polysaccharides were purified from the culture liquids of two fungal strains of this genus: a heteropolysaccharide composed of galactose, glucose and mannose, and a high-molecular weight, O-6 branched β-(1 → 3)-d-glucan. The β-glucans of both endophytes had antiproliferative action against the tumor cell lines, although this
Conflict of interest
The authors declare that there is no conflict of interests.
Acknowledgments
The authors are grateful to Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq; 311534/2014-7 and 447265/2014-8), Fundação Araucária (276/2014) and Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP; 05/53879-3) for financial support. R.C. Orlandelli thanks Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for the doctoral scholarship.
References (28)
A.F.D. Vasconcelos et al.Three exopolysaccharides of the β-(1→6)-d-glucan type and a β-(1→3;1→6)-d-glucan produced by strains of Botryosphaeria rhodina isolated from rotting tropical fruitCarbohydr. Res.(2008)
J. Chen et al.Medicinal importance of fungal β-(1-3),(1-6)-glucansMycol. Res.(2007)
J.A. Bohn et al.β-D-Glucans as biological response modifiers: a review of structure-functional activity relationshipsCarbohydr. Polym.(1995)
A.R.A. Pires et al.Cytotoxic effect of Agaricus bisporus and Lactarius rufus β-d-glucans on HepG2 cellsInt. J. Biol. Macromol.(2013)
R.C. Orlandelli et al.Screening of endophytic sources of exopolysaccharides: preliminary characterization of crude exopolysaccharide produced by submerged culture of Diaporthe sp. JF766998 under different cultivation timeBiochim. Open(2016)
M.M. BradfordA rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye bindingAnal. Biochem.(1976)
I. Ciucanu et al.A simple and rapid method for the permethylation of carbohydratesCarbohydr. Res.(1984)
M.M. Corsaro et al.Chemical structure of two phytotoxic exopolysaccharides produced by Phomopsis foeniculiCarbohydr. Res.(1998)
A.C. Ruthes et al.d-Glucans from edible mushrooms: a review on the extraction, purification and chemical characterization approachesCarbohydr. Polym.(2015)
S.K. Bhanja et al.Isolation and characterization of the immunostimulating β-glucans of an edible mushroom Termitomyces robustus varCarbohydr. Res.(2012)
M.L. Corradi da Silva et al.Structural characterization of the cell wall d-glucans isolated from the mycelium of Botryosphaeria rhodina MAMB-05Carbohydr. Res.(2008)
References